Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults, accounting for 25-30% of all leukemias in the United States. During the past decade, there has been a paradigm shift in CLL treatment, with increasing adoption of novel oral agents (NOAs) such as acalabrutinib (ACALA), duvelisib (DUV), ibrutinib (IBR), idelalisib (IDELA), and venetoclax (VEN) instead of traditional chemoimmunotherapy (CIT). Unlike CIT, most NOAs are given daily for an indefinite period of time and are self-administered at home, raising concerns about adherence and discontinuation. The discontinuation patterns of NOAs in a real-world population of CLL patients is currently unknown.
Methods
Using the Veterans Administration (VA) Cancer Registry System and electronic healthcare records, we identified patients treated for CLL with NOAs in the VA from November 1, 2013 to November 30, 2018. Patients were followed from the first NOA dispensation until death or the end of the study observation (May 31, 2019). NOAs were selected in accordance with the National Comprehensive Cancer Network (NCCN) guidelinesfor CLL and were extracted using pharmacy dispensation records.
Discontinuation was defined as the absence of NOA dispensation within 60 days of estimated exhaustion of patient's NOA supply from the last recorded dispensation. A discontinuation event for each NOA treatment episode in each patient was classified as either 1) a drug holiday, if there was evidence of resumption of the same treatment without any other treatment interventions after the first discontinuation; or 2) permanent discontinuation, in which the treatment ceased without evidence of resumption for 60 days or there was evidence of new treatment initiation after the discontinuation of previous treatment. We report the proportion of discontinuation and exposure-adjusted discontinuation rates (e-AR), which were calculated using the length of NOA duration in person-time.
Results
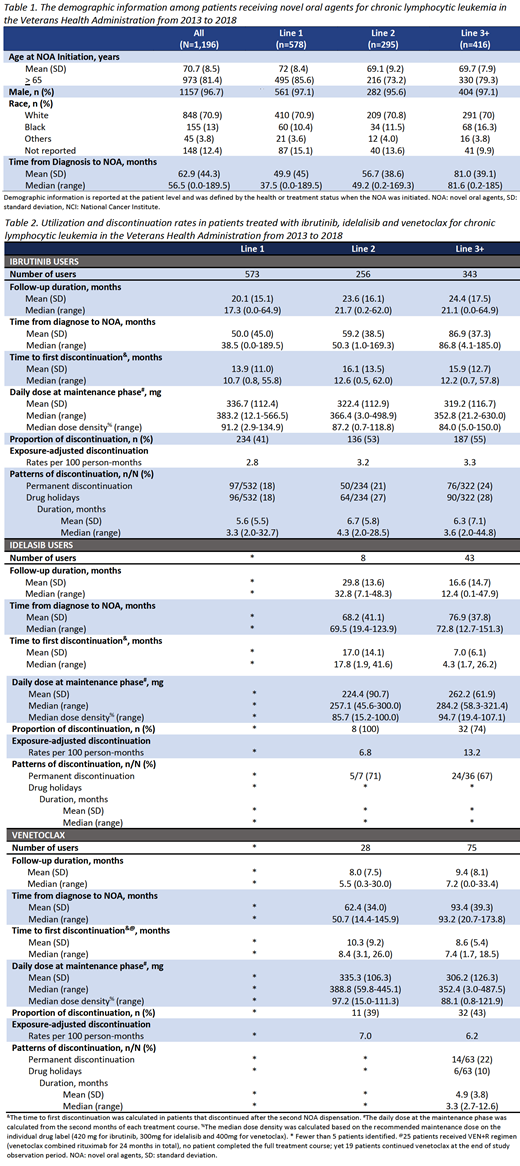
We identified 1,196 CLL patients treated with NOAs from November 1, 2013 to November 30, 2018. The mean age at NOA initiation was 70.7 years; 96.7% of patients were male. The median time from diagnosis to NOA initiation was 56.5 months (0-189.5 months). Of the 1,196 patients treated with NOAs, 1,172 received IBR, 53 received IDELA, and 106 received VEN. There were few patients (<10)treated with ACALA and DUV, therefore these patientswere omitted from the final analyses.
During a median follow-up of 18.9 months (0-65.3 months), 48.2% NOA treatment courses were followed by a discontinuation event. The proportions of patients who discontinued for IBR, IDELA, and VEN were 47.5%, 77.4%, and 41.5%. The e-AR of IBR, IDELA, and VEN are reported in Table 2.
At the end of the study observation (May 2019), 52.6% of IBR, 22.6% of IDELA, and 58.3% of VEN treatment courses were still being administered. In first-line (L1) IBR, the median NOA treatment duration until the first discontinuation was 10.7 months (0.8-55.8 months), 12.6 months (0.5-62.0 months) in second-line (L2) IBR, and 12.2 months (0.78-57.8 months) in third-line or subsequent lines (L3+) IBR. In IDELA treatment courses, the median treatment duration until the first discontinuation was 17.8 months (1.9-41.6 months) in L2 and 4.3 months (1.7-26.2 months) in L3+. In VEN, the median treatment duration until the first discontinuation was 8.4 months (3.1-26.0 months) in L2 and 7.4 months (1.7-18.5 months) in L3+.
Of 532 L1 IBR treatment courses with >1 dispensation, 96 (18%) were associated with a drug holiday and 97 (18%) with permanent discontinuation. These numbers were 64 (27%) and 50 (21%) for L2 IBR courses, and 90 (28%) and 76 (24%) for L3+ IBR courses. Similarly, 24 (67%) of L3 IDELA treatment courses with >1 dispensation were associated with permanent discontinuation. Among 63 L3+ VEN courses with >1 dispensation, 6 (10%) were associated with a drug holiday and 14 (22%) with permanent discontinuation.
Conclusions
To our knowledge, this study is the first to report on the NOA discontinuation in a nationwide VA cohort of CLL patients treated in a real-world setting. Our results suggest there is a substantial proportion of drug holidays and permanent discontinuation among commonly used NOAs. Further efforts will focus on understanding factors leading to discontinuation and the impact of discontinuation/drug holidays on clinical outcomes.
Biondo:Genentech, Inc.: Employment; F. Hoffmann-La Roche Ltd: Equity Ownership. Halloran:Roche: Equity Ownership; Genentech, Inc.: Employment. Shapouri:Roche: Equity Ownership; Genentech, Inc.: Employment. Wu:Genentech, Inc.: Employment. Sauer:University of Utah and SLC VA Medical Center: Employment. Halwani:Miragen: Research Funding; Kyowa Hakko Kirin: Research Funding; Pharmacyclics: Research Funding; Amgen: Research Funding; Seattle Genetics: Research Funding; Genentech, Inc.: Research Funding; AbbVie: Research Funding; Takeda: Research Funding; Bristol-Myers Squibb: Research Funding; Immune Design: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal