Introduction: While treatments for CLL have improved in recent years, CLL remains incurable for most patients who often rely on long-term suppressive medications. These can present complications associated with undesirable side effects and the risk of relapse. NK cell therapy holds great promise due to NK cells' powerful innate anti-tumor effects, with the potential to induce deep remission or even cure. However, previous efforts have been constrained by low cell numbers and limited cytotoxicity against CLL cells. Stimulating NK cells ex vivo with K562-based feeder cells expressing membrane-bound IL-21 (mbIL-21) induces high levels of expansion and activation, with potent cytotoxicity against various tumor cells (Denman et al. PLoS ONE 2012).
We have recently demonstrated that allogeneic NKs from normal donors, expanded using mbIL-21, are potently cytotoxic to CLL cells (Yano et al. iwCLL 2019). Here, we test this technique with autologous NK cells. Autologous therapy will allow the benefits of administering activated NK cells without the risks of immunosuppression required for allogeneic treatment.
Methods: We isolated NK cells from CLL patient blood and expanded them for 21 days using IL-21 expressing feeder cells and IL-2 (Denman et al. PLoS ONE 2012). We then characterized the cytotoxic capacity of the CLL-derived expanded NKs (CLL-XNKs) using calcein release assays. We measured both direct cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) against OSU-CLL and Mec1 CLL cell lines, allogeneic primary CLL cells, and autologous CLL cells. We compared CLL-XNK cells to unstimulated NK cells from normal donors and to normal donor-derived expanded NKs (ND-XNKs), produced using the same protocol.
Results: During mbIL-21 NK stimulation, CLL-derived NK cells underwent an average of 5,900-fold expansion and maintained exponential growth throughout the 21-day expansion. This growth is similar to normal donor-derived NK cells (doubling time 1.6 days for CLL-derived vs. 1.5 days for donor-derived, p = 0.26, n=5).
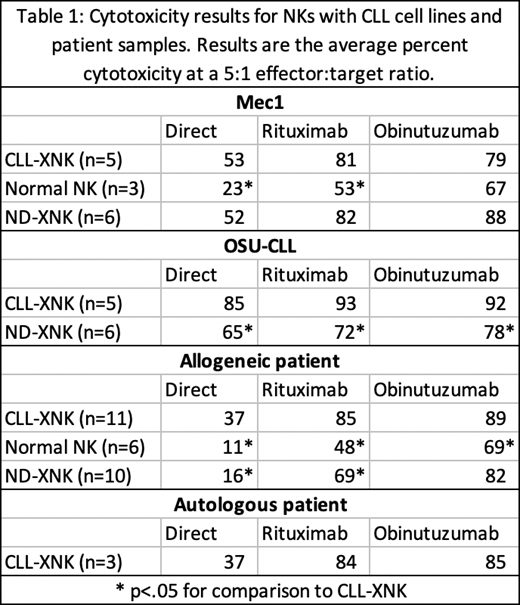
Cytotoxicity data comparing CLL-XNK cells versus ND-XNKs and unstimulated donor-derived NK cells is included in Table 1. We tested a range of effector:target ratios and found a dose response pattern of increasing cytotoxicity from 0.3125:1 to 10:1 ratios. Interestingly, while ADCC with either antibody was superior to direct cytotoxicity, obinutuzumab was not superior to rituximab for stimulating CLL-XNK cytotoxicity, differing from our experiences with ND-XNKs (Yano et al. iwCLL 2019).
First, we demonstrate that CLL-XNK cells show potent cytotoxicity against both OSU-CLL and Mec1 CLL cell lines, via both direct cytotoxicity and ADCC (Table 1). These results show CLL-XNK cells to be similar or greater in potency in comparison to both ND-XNKs and normal unstimulated NKs.
CLL-XNK cells also show cytotoxicity against allogeneic primary CLL cells, with greater cytotoxic activity than unstimulated, normal donor-derived NK cells (Table 1). Interestingly, while ADCC with obinutuzumab was similar between CLL-XNKs and ND-XNKs (p=.44), direct cytotoxicity and rituximab-induced cytotoxicity were both higher with CLL-XNKs than ND-XNKs (p=.0001 and .041) (Table 1). These results contrast with previous reports that LAKs derived from CLL patients have decreased potency (Foa et al. and Santiago-Schwarz et al. Blood 1990).
Finally, CLL-XNKs showed potent cytotoxicity against autologous CLL cells using both direct cytotoxicity and ADCC (Table 1).
Conclusion: We have successfully expanded NK cells from CLL patients and demonstrated their cytotoxicity against CLL cell lines, unmatched CLL cells, and autologous CLL cells. These patient-derived cells are superior to normal unstimulated NKs and are similar or even better than expanded donor NK cells. Ongoing studies will explore in vivo function of these NK cells, combination with CLL-targeted treatments, and further functional measures. IL-21-expanded NK cells represent a promising new therapy for CLL in both allogeneic and autologous settings.
(*MY and JRL contributed equally to this work. MY is a recipient of a Pelotonia Graduate Fellowship and JRL is a recipient of a Hendrix Summer Scholars Fellowship. This work was supported by NIH R35 CA197734.)
Lee:Kiadis Pharma: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Muthusamy:Ohio State University: Patents & Royalties: OSU-2S. Byrd:Novartis: Other: Travel Expenses, Speakers Bureau; BeiGene: Research Funding; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Acerta: Research Funding; Ohio State University: Patents & Royalties: OSU-2S; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal