BACKGROUND:
Recent prospective randomized phase III studies that include targeted agents in first-line (1L) chronic lymphocytic leukemia (CLL) all demonstrate major improvements in progression-free survival (PFS) compared to chemoimmunotherapy, with no or minimal impact on overall survival (OS). PFS has generally been a poor predictor of OS in indolent lymphoma and CLL.
The clinical trial results with 1L targeted therapy including ibrutinib and venetoclax (in combination with obinutuzumab) may raise questions regarding the role of chemoimmunotherapy (bendamustine-rituximab and fludarabine-cyclophosphamide-rituximab) in the management of CLL. However, high cost of 1L targeted therapy limits its global availability. We recently found that time to second treatment (TT2T), rather than time to first treatment (TT1T), was a good predictor of OS in patients with follicular lymphoma.
We sought to test the hypothesis that TT2T is correlated with OS in CLL and determine if there are pre-treatment variables that help determine which patients would benefit more from early use of targeted agents, and which patients can be managed with time-limited, relatively cost-effective chemoimmunotherapy.
METHODS:
We retrospectively identified 1974 patients with CLL diagnosed at MSKCC between January 1998 and December 2014. To undertake this analysis, we examined a subset of 298 patients with CLL/SLL who had complete data to determine the CLL-International Prognostic Index (CLL-IPI). The 298 patients with complete data had outcomes similar to that of patients in the CLL-IPI cohort. TT1T and TT2T were calculated from time of disease diagnosis to start of first and second treatments, respectively. Median time to event was calculated using the Kaplan-Meier method for each event: TT1T, TT2T and OS. Univariate and multivariate ordinal logistic regression models were constructed to identify risk factors (RF) for requiring systemic therapy. Patients were characterized by the number of CLL-directed treatments: 0 vs 1 vs ≥2 treatments. We then evaluated OS and TT2T based on the identified RF, using the log rank test to compare outcomes.
RESULTS:
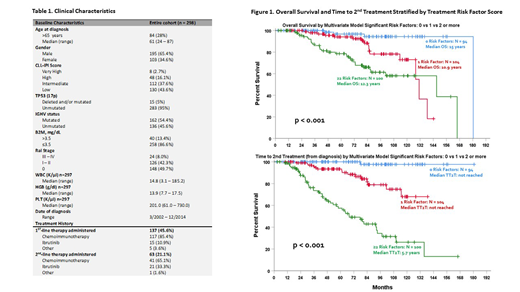
We identified 298 patients with CLL/SLL that had complete data to determine the CLL-IPI. Patient characteristics are shown in Table 1. There was a male predominance (1.89:1), 28% were above age 65 with a median age of 61 years (range 24-87), and CLL-IPI score was Low and Intermediate to Very High in 43.6% (130/298) and 56.4% (168/298), respectively.
At a median follow-up of 6.6 years: median TT1T was 6.5 years, median TT2T was not reached, and median OS was 13.8 years. TT2T was superior to TT1T for predicting OS. After univariate and multivariate analyses, 3 clinical factors were found to independently predict the requirement for systemic therapy: IGHV unmutated (OR 5.331, 95% CI 3.09 - 9.20, p<0.001), elevated B2M (OR 3.70, 95% CI 1.40 - 9.77, p=0.008) and advanced Rai staging (OR 11.36, 95% CI 1.97 - 65.46, p=0.007). Overlapping 95% confidence intervals allowed for these 3 RF to be combined to create a simple prognostic model, defined as 0 vs 1 vs ≥2 of the 3 predictive RF. This model was predictive for both TT2T and OS. Patients with 0 RF had superior OS (p < 0.001) and had a longer time before needing 2L therapy (p < 0.001), Figure 2.
DISCUSSION:
These data support the conclusion that TT2T is a surrogate for OS in CLL/SLL in this group of patients treated largely with 1L chemoimmunotherapy. Patients with 0 or 1 RF have a significantly longer TT2T and OS compared to patients with 2 or more RF, suggesting that 1L chemoimmunotherapy provides more durable responses for patients with 0 and 1 RF than for patients with 2 or more RFs. For patients with durable responses, chemoimmunotherapy provides a time limited strategy with prolonged treatment-free period prior to the initiation of 2L therapy. The absence of an OS benefit with targeted 1L therapy in the ALLIANCE A041202, CLL14 and iLLUMINATE trials supports delaying targeted therapy to 2L. In contrast, patients with ≥2 RF have a median TT2T of 5.7 years, significantly shorter than that of patients with 0-1 RF (median not reached). Given the limited benefit of 1L therapy in patients with ≥2 RF in this predominantly chemo treated group, it would be appropriate to evaluate if utilizing 1L targeted therapy would be superior to chemoimmunotherapy. Additional analyses are ongoing to determine if these findings can be validated in an independent cohort.
Soumerai:TG therapeutics: Research Funding; BeiGene: Research Funding; BostonGene: Research Funding; AbbVie: Consultancy; Verastem: Consultancy; Genentech/Roche: Research Funding. Roeker:AbbVie: Equity Ownership; Abbott Laboratories: Equity Ownership. Mato:DTRM Biopharma: Research Funding; Genentech: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Gilead: Research Funding; LOXO: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB member , Research Funding; Celgene: Consultancy; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Acerta: Consultancy; Janssen: Consultancy. Zelenetz:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal