Background: Oral fludarabine and intravenous rituximab (FR) was the standard first-line therapy for CLL or small lymphocytic lymphoma (SLL) patients (pts) in BC from 2003-2015. Ibrutinib for relapsed/refractory (R/R) CLL was introduced and publicly funded in 2015. Our aim was to review long term outcomes of all CLL/SLL pts treated with FR in BC, including the impact of 2nd line therapy with ibrutinib versus chemoimmunotherapy and to report the risk of secondary malignancies in this population based cohort.
Methods: The BC Provincial CLL Database was used to identify all CLL/SLL pts who received first-line FR from 2003-2017. The BC Cancer Registry was used to identify secondary malignancies occurring after FR. Primary outcomes were overall survival (OS) and treatment free survival (TFS), defined as start of FR to next-line therapy or death/last follow-up. Variables examined for impact on OS/TFS included age at FR, gender, primary diagnosis (CLL vs SLL), B symptoms, advanced stage (Rai stage 3-4 CLL, Ann Arbor 1-2 SLL), baseline hemoglobin, lymphocyte count, platelets, LDH and FISH abnormalities. All variables significant on univariate analyses (P<.1) were included in multivariate Cox proportional hazard regression models to identify significant predictors of OS/TFS.
Results: 673 pts were identified as receiving FR as first-line therapy for CLL (86%) or SLL (14%). Median time from CLL/SLL diagnosis to FR was 2.5 years (y) (range 0.1-27.3). Median age at FR was 67 y (range 26-91) with 73% ≥ 60 y and 39% ≥ 70 y. Most pts were male (66.1%), had early stage disease (84.2%) with no B symptoms (89.7%) and normal LDH (81.1%). Of 411 pts with pre-treatment FISH testing, prevalence of FISH abnormalities were: 48.5% del13q, 25.7% trisomy 12, 12.9% del11q, 8.0% del17p. Median number of FR cycles was 6 (range 1-10).
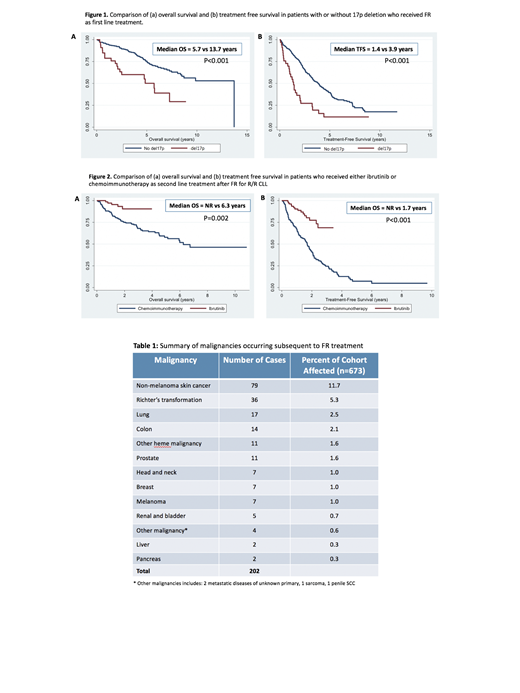
Median follow-up of living pts from FR was 6.4 y (range 0.2-12.7). 2 y and 5 y OS were 89.4% (95% CI: 86.8-91.6) and 73% (95% CI: 69.0-76.6) respectively; median OS 11.6 y (95% CI: 4.6-13.7 y). 2 y and 5 y TFS were 72% (95% CI: 68-75%) and 37% (95% CI: 33 - 41) respectively, median TFS 3.8 y (95% CI: 1.78-7.09). Those with del17p had significantly worse OS and TFS compared to those without (median OS 5.7 vs 13.7 y, P<.001; median TFS 1.4 vs 3.9 y, P<.001), Fig. 1. Multivariate analysis identified only del17p (HR 4.35, 95%CI: 2.10-9.01, P<.001) and age at FR (HR 1.04, 95% CI: 1.01-1.07, P=.007) as significant predictors of OS, and del17p (HR 4.3, 95% CI: 2.5-7.5, P<.001) as a significant predictor of TFS.
During the follow up period, 351 pts (52%) went on to 2nd-line therapy: ibrutinib 87 (including 2 with BR and 1+R), cyclophosphamide-based (CVP/CHOP) +/- R 102, repeat FR 71, FCR 6, F alone 21, bendamustine +/-R 13, chlorambucil+/-R 38, steroids 3, R alone 3, alemtuzumab 2, other chemotherapy 3 and allotransplant 2. Median follow-up after 2nd-line therapy was 2.8 y (range 0.1-10.8). Median OS and TFS from 2nd-line treatment (TFS2) for ibrutinib (n=87) vs. for other treatments (n=264) was: OS not reached vs 5.3 y, P<.001; TFS2 not reached vs 1.2 y, P<.001. These significant differences persisted when analyses were restricted to those who received ibrutinib vs. chemoimmunotherapy (n=169): median OS not reached vs. 6.3 y (P=.002); median TFS not reached vs. 1.7 y (P<.001), Fig. 2. 2 y OS and TFS2 after ibrutinib were 91% (95% CI: 80-96%) and 78% (95% CI: 65-87%), respectively.
A total of 202 malignancies were recorded after initiation of FR in 166 pts (24.7%), Table 1. The median time from FR to 2nd malignancy was 2.3 y (range 0.1-13.5). Richter's transformation (RT) occurred in 36 pts (5.3%) at median 1.9 y (range 0.1-13.2) from FR. Most frequent 2nd malignancies were: non-melanoma skin cancer (11.7%), lung (2.5%), colon (2.1%), other heme (1.9%), and prostate (1.8%). There were 4 cases of acute myeloid leukemia (AML), 2 of which received alkylator therapy after FR prior to AML diagnosis.
Conclusions: In this large, homogeneous cohort of CLL/SLL pts treated with first-line FR, including nearly 40% of pts ≥ age 70, we demonstrate a short median TFS of 3.8 y; however, a long OS of 11.6 y. Rates of 2nd malignancies are low after this non-alkylator based chemoimmunotherapy regimen. Ibrutinib for R/R CLL/SLL after FR resulted in significantly improved survival over alternate therapy, with excellent 2 yr OS 91% and TFS 78%. These data demonstrate the efficacy of FR and the benefit of ibrutinib over chemoimmunotherapy as second-line therapy for CLL/SLL in the real-world.
Bruyere:Jenssen: Other: Travel Grant; Celgene: Honoraria. Villa:Roche, Abbvie, Celgene, Seattle Genetics, Lundbeck, AstraZeneca, Nanostring, Janssen, Gilead: Consultancy, Honoraria. Scott:Celgene: Consultancy; Roche/Genentech: Research Funding; Janssen: Consultancy, Research Funding; NanoString: Patents & Royalties: Named inventor on a patent licensed to NanoSting [Institution], Research Funding. Savage:BMS, Merck, Novartis, Verastem, Abbvie, Servier, and Seattle Genetics: Consultancy, Honoraria; Seattle Genetics, Inc.: Consultancy, Honoraria, Research Funding. Connors:Takeda Pharmaceuticals: Honoraria; Seattle Genetics: Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy. Sehn:TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria. Gerrie:Lundbeck, Seattle Genetics: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal