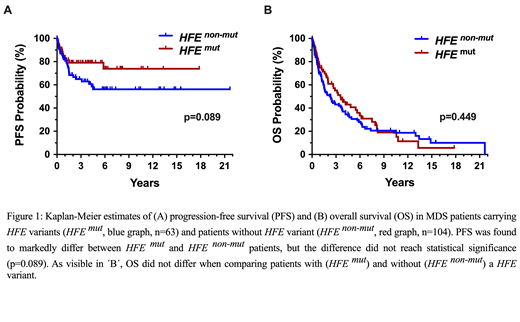
Myelodysplastic syndromes (MDS) are myeloid neoplasms defined by peripheral cytopenia, dysplasia of myeloid cells and an increased risk to transform to secondary acute myeloid leukemia (AML). Although several laboratory and molecular risk factors and scoring systems have been established, little is known about genetic features contributing to disease evolution, AML development, and survival. Accumulating evidence suggests that iron metabolism and transfusion frequency are important prognostic determinants in MDS. It has also been described that patients with MDS frequently display HFE gene variants. However, little is known about the clinical impact of HFE variants. We examined the HFE status by restriction fragment length polymorphism (RFLP) analysis covering 2 common variants (H63D and C282Y) in 167 patients with MDS (observation-period: 1992-2019) and in 494 healthy Austrian controls. The median age of our MDS patients at diagnosis was 69 years (range 27-85 years) with a f:m ratio of 1:1.13. In the MDS cohort the international prognostic scoring systems, IPSS and IPSS-R, were applied, and in 94 patients a ´myeloid mutation´ screen was performed by next-generation sequencing (NGS). In all patients, serum ferritin levels were recorded at diagnosis and during follow up. Variant HFE species (H63D and/or C282Y) were detected in 63/167 patients with MDS (38.4%) and in 170/494 controls (34.4%). Median ferritin levels at diagnosis were slightly higher in HFE-mutated patients (409 mg/dL; range: 23-7415 mg/dL) compared to non-mutated patients (346.5 mg/dL; range: 10-5450 mg/dL) (p=0.62). During the first 2 years of transfusion therapy, HFE-mutated patients showed a slightly faster increase in serum ferritin levels compared to non-mutated patients. However, again, the difference was not significant, and patients with massive iron overload were found in both cohorts. When examining French-American-British (FAB) subgroups of MDS, the percentage of patients withHFE variants was higher in the group with refractory anemia, RA (22/53=41.5%) or RA with ring sideroblasts, RARS (17/39=43.9%) than in the group of RA with excess of blasts, RAEB (16/46=34.8%) or RAEB in transformation (5/17=29.4%). In CMML, 5/12 patients (41.7%) were found to carry a HFE variant. Clear differences were also observed between subgroups of MDS patients classified by World Health Organization (WHO) criteria. The prevalence of HFE mutations was 50.0% (13/26) in patients with ring sideroblasts (RS) and multilineage dysplasia (MDS-RS-MLD), 50.0% in MDS-MLD (6/12), 43% (7/18) in MDS with RS and single-lineage dysplasia (MDS-RS-SLD), 42.9% (12/28) in patients with excess of blasts-1 (MDS-EB1), 38.9% (7/18) in MDS-SLD, 21.4% (6/28) in MDS-EB2, and 20% (2/10) in MDS with isolated 5q-. When splitting patients according to the IPSS and IPSS-R, patients at highest risk were found to have a lower prevalence of HFE variants (IPSS high: 20%; IPSS-R very high: 15.4%) compared to other risk groups (IPSS low: 34.9%, int-1: 42.0%, int-2: 31.0%; and IPSS-R very low: 33.3%, low: 38.5%, int: 36.8%, high: 42.9%). Next, we examined correlations between HFE variants and somatic myeloid mutations in our MDS patients (n=94). No significant correlations were found when comparing expression of HFE variants with somatic mutations in ASXL1, ASXL1, SF3B1, SRSF2, TET2, DNMT3A, RUNX1, NRAS, TP53, IDH2 or EZH2 detected by NGS. Finally, we examined the HFE status in the context of clinical end points. In these studies, we found a clear difference in AML-free survival between HFE-mutated and non-mutated patients, although the difference did not reach statistical significance (p=0.089) (Figure 1A). No differences in overall survival (OS) were found when comparing patients with or without HFE variants (Figure 1B). In conclusion, the HFE variants H63D and C282Y are frequently detected in Austrian patients with MDS. These variants are more commonly detectable in low risk FAB patients and correlate roughly with higher ferritin levels at diagnosis and during transfusion therapy. In addition, HFE-mutated MDS patients have a slightly better AML-free survival. However, the correlation is weak. Therefore, we currently do not recommend screening for HFE gene variants at diagnosis in patients with MDS unless ferritin levels are excessively high or increase very rapidly during transfusion suggesting the possibility of a concomitant primary hemochromatosis.
Valent:Blueprint: Research Funding; Celgene: Honoraria; Deciphera: Honoraria, Research Funding; Pfizer: Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Sperr:Novartis: Honoraria; Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal