Background: Hypomethylating agents (HMAs) are used to treat patients with lower-risk myelodysplastic syndrome (LR-MDS) relapsing after the use of hematopoietic cytokines or presenting initially with more than two lineages of cytopenias. However, the significance of underlying genetics and allelic burden changes after HMAs are still under investigation. This study investigated the effects of allelic burden changes on the long-term outcomes in LR-MDS patients treated with HMAs.
Methods: This study included 61 patients with LR-MDS treated with azacitidine. Bone marrow samples were taken at diagnosis and follow-up after median 4 cycles. Targeted deep sequencing on a custom myeloid gene panel of 84 genes (Agilent SureSelect) was performed on trios of T-cell, pre-HMA, and post-HMA samples on 61 LR-MDS patients. Patients were divided into groups according to the post-HMA variant allele frequency (VAF): low-VAF (< 2%), high-VAF (≥ 2%), and no-mutation group (absence of mutations at diagnosis and follow-up). Overall survival (OS) was defined as the time from the azacitidine treatment until death from any cause, which was analyzed using the Kaplan-Meier method and the groups were compared using the log-rank test. The cumulative incidence of AML was calculated using the Gray method, considering death without AML as a competing risk. Fine-Gray proportional hazard regression with a competing event was used to identify risk factors for the incidence of AML.
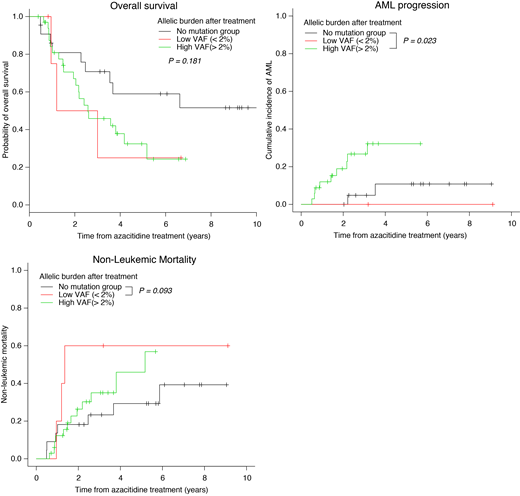
Results: Median age was 67 years (range 31-81), and 41 patients (67%) were male. IPSS risk group were low in 2 patients (3%) and intermediate-1 in 59 (97%). At diagnosis, 38 patients harbored at least one mutation. Most frequently mutated genes were ASXL1 (n=11, 18%), TET2 (n=10, 16%), and SRSF2 (n=7, 11%) followed by RUNX1, SF3B1, U2AF1, IDH2, and DNMT3A. Azacitidine was administered median 8 cycles (range 2-44). The overall response (CR, PR, HI) was achieved in 18 patients (30%). With median follow-up duration of 31 months (range 4.7-135 months), leukemic transformation occurred in 11 patients (18%). Mutational allelic burdens were decreased from median 20.9% (range 0.1-67.2) to 11.0% (range 0.0-74.9%). At follow-up, 5 patients were low-VAF group and 33 were high-VAF group. OS rate was not different between the low-VAF and high-VAF group (50% vs 46% at 3 years; p=0.80). Three-year cumulative incidence of AML was higher in high-VAF group compared to low-VAF (0%) and no-mutation group (4.8%, p=0.02). However, non-leukemic mortality was higher in low-VAF group than no-mutation group (60% vs 23%, p=0.09), which explains similar OS rate between low-VAF and high-VAF group. In the multivariate analysis, high-VAF was an independent predictive factor for an AML transformation in LR-MDS patients treated with azacitidine (HR 5.20, p=0.04).
Conclusion: The current study showed that the high residual allelic burden is associated with an increased AML transformation in LR-MDS patients treated with azacitidine, irrespective of the clinical response. The higher non-leukemic mortality explains inferior OS in low-VAF group compared to no-mutation group.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal