Background
Hypomethylating agents (HMAs) are the current standard of care therapy in high-risk MDS. However, only ~50% of patients with MDS respond to HMAs and most responding patients eventually progress. Outcomes after HMA failure in patients with high-risk MDS are especially poor, with median survival of 4 to 6 months. Venetoclax in combination with HMAs led to deep and durable remissions in elderly patients with newly diagnosed AML with CR+CRi rate of 67% and median survival of 17.5 months.Here, we performed a multicenter retrospective analysis to determine outcomes in patients with MDS receiving HMA + venetoclax (off-label) and identified risk factors associated with response and survival.
Methods
After obtaining institutional review board approval(s), we retrospectively reviewed charts of patients receiving HMA and venetoclax between January 1, 2018 to July 15, 2019. Criteria for inclusion were a pathologically confirmed diagnosis of MDS and treatment with either decitabine or azacitidine in combination with venetoclax. Patients with progression to AML prior to treatment with HMA+ venetoclax were excluded. Bone marrow response assessments were evaluated per WHO 2006 criteria. For subjects who required a delay in study treatment for blood count recovery after bone marrow evaluation, hematology values for up to 2 weeks were used to determine response. When available, cytogenetics, next generation sequencing, and flow cytometry data were included for analysis. HMA failure was defined as progression to a higher IPSS-R risk MDS while on HMA or lack of response after 4 cycles of HMA treatment. On univariate analysis, Fisher's exact test and Student t tests were used for overall response and Kaplan-Meier estimates were used to summarize OS and RFS.
Results
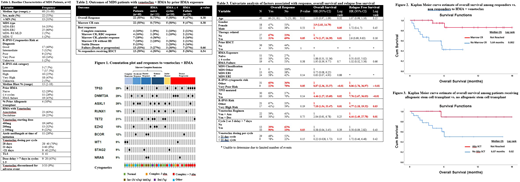
42 patients met criteria for inclusion. The patient population was predominantly male, elderly, R-IPSS very poor cytogenetics, R-IPSS very high risk, MDS-EB2, and with HMA failure prior to initiation of HMA + venetoclax (Table 1.) Median blast count was 13% (range 2-18%). MDS was classified as therapy-related (t-MN) in 13 (31%) patients. TP53 mutations with complex karyotype occurred in 9 out of 34 (26%) patients (Figure 2). Azacitidine was the most common HMA used in combination with venetoclax (63%). Median follow up from the start of the combination was 5.4 months. Out of 40 patients evaluable for response, the ORR and marrow CR rate was 55%, median RFS and OS were not reached. Patients who achieved a marrow CR had a significantly prolonged OS in comparison to non-responders (median OS not reached vs. 5.64 months, p= 0.002). Among HMA naïve patients, the marrow CR rate was 73%. Furthermore, the marrow CR rate was 47% for patients with HMA failure and there was no significant difference in response or survival based on HMA exposure (Table 2). Median time to initial response was 1.6 months. On univariate analysis, therapy related disease and very poor risk cytogenetics were significantly associated with decreased response and a delay in starting cycle 2 or 3 was associated with increased response. Female gender, t-MN, very poor risk cytogenetics and a TP53 mutation were significantly associated with inferior OS. Very poor risk cytogenetics, a TP53 mutation, and the decitabine combination was significantly associated with decreased RFS (Table 3). Of note, neither a dose delay of venetoclax > 7 days or <21 day cycle of venetoclax were associated with decreased median OS or RFS. Out of 22 responders, 13 (59%) received allogeneic stem cell transplantation (allo-SCT). Allo-SCT was associated with increased median OS (not reached vs. 6.07 months, p=0.02, Figure 3.) and RFS (not reached vs. 3.51 months, p<0.01).
Conclusion
In summary, venetoclax in combination with HMAs led to high rates of marrow remission (55%) and hematologic improvement (38%) in a very high risk and heavily treated MDS population. The response rate was higher in HMA naïve patients but not significantly decreased among patients with HMA failure. Achievement of marrow CR led to significantly prolonged OS. HMA + venetoclax also led to allo-SCT in 59% of all responders and 56% of responders with HMA failure. Complex karyotypes and TP53 mutations were associated with worse response and survival. These results suggest that adding venetoclax may salvage patients failing to respond optimally to HMA, thus allowing more patients to proceed to allo- SCT, and likely warrant testing prospectively.
Stein:Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma US, Inc: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Bioline: Membership on an entity's Board of Directors or advisory committees. Tallman:ADC Therapeutics: Research Funding; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; UpToDate: Patents & Royalties; Biosight: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; UpToDate: Patents & Royalties; Cellerant: Research Funding; Cellerant: Research Funding; Cellerant: Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellerant: Research Funding; ADC Therapeutics: Research Funding; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biosight: Research Funding; Cellerant: Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellerant: Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; Biosight: Research Funding; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties. Gill:Novartis: Research Funding; Tmunity Therapeutics: Research Funding; Carisma Therapeutics: Research Funding; Amphivena: Consultancy; Aro: Consultancy; Intellia: Consultancy; Sensei Bio: Consultancy; Carisma Therapeutics: Equity Ownership. Koprivnikar:Amgen: Speakers Bureau; Pfizer: Honoraria; Abbvie: Speakers Bureau; Novartis: Speakers Bureau. Sallman:Incyte: Speakers Bureau; Celyad: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding, Speakers Bureau; Jazz: Research Funding; Novartis: Speakers Bureau; Abbvie: Speakers Bureau. Goldberg:ADC Therapeutics: Research Funding; American Society of Clinical Oncology: Research Funding; American Society of Hematology: Research Funding; Celgene: Consultancy; Daiichi-Sankyo: Consultancy, Research Funding; Pfizer: Research Funding; DAVA Oncology: Honoraria; Abbvie: Research Funding; Arog Pharmaceuticals: Research Funding; Abbvie: Consultancy. Komrokji:Novartis: Speakers Bureau; pfizer: Consultancy; DSI: Consultancy; Incyte: Consultancy; Agios: Consultancy; JAZZ: Speakers Bureau; celgene: Consultancy; JAZZ: Consultancy.
We will describe outcomes for patients receiving venetoclax in combination with hypomethylating agents in MDS.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal