Introduction: P53 is negatively regulated by MDM2 and increased expression of MDM2 in PV and MF CD34+ cells and megakaryocytes has been observed. Recently, we have shown that MDM2 antagonists termed nutlins can selectively eliminate MPN CD34+ cells (1-2). We have reported a phase 1 trial of PV patients treated with an oral nutlin, RG7388, which resulted in hematological responses and an improvement in symptoms in a substantial subset of PV patients intolerant or refracting to frontline therapy (3). Nutlins, however only target malignant cells with wild type p53, cannot completely eliminate the MPN stem cell pool and are associated with gastrointestinal toxicity. We therefore searched for another class of compounds to combine with nutlins to more effectively eliminate MF stem cells. Recently, an oral imipridone compound, ONC201, has been evaluated in clinical trials of patients with hematological malignancies (leukemia/lymphoma) and solid tumors. ONC201 was discovered by screening drug libraries for compounds that transcriptionally induced the TNF-related apoptosis inducing ligand (TRAIL) which triggers apoptosis through the extrinsic pathway which is independent of p53. ONC201 is also as potent allosteric agonist of mitochondrial caseinolytic protease P (ClpP) (4). Hyperactivation of ClpP by ONC201 increases mitochondrial proteolysis and leads to mitochondrial dysfunction and triggers the extrinsic apoptotic pathway. We hypothesized that combination treatment with nutlin (RG7112/RG7388) and ONC201 might provide an effective therapeutic strategy to selectively induce apoptosis of MF CD34+ cells by activating both intrinsic and extrinsic apoptosis pathways.
Results: We tested whether RG7112 combined with ONC201 was capable of specifically targeting MF CD34+ cells. Treatment with ONC201 alone decreased total hematopoietic colonies generated by MF CD34+ cells in a dose dependent fashion. Treatment with ONC201 at dose of 10 uM decreased MF colony numbers by 50%, and a 500nM dose of RG7112 decreased MF colony numbers by 30%. Combination therapy with RG7112 and ONC201, however, decreased MF colony numbers by 70% (ONC201 vs RG-ONC201: p=0.005 and RG vs RG-ONC201: p=0.001, respectively). By contrast, treatment with these agents alone or in combination did not decrease colony formation by normal CD34+ cells. ONC201 or RG7112 alone at the doses utilized did not alter the genotype of the MF colonies; however, combination therapy decreased the numbers of JAK2V617F+ colonies by 20%, and increased wild type colony numbers by 24%. These data suggest that combination treatment with RG7112 and ONC201 can specifically deplete MF malignant CD34+ cells.
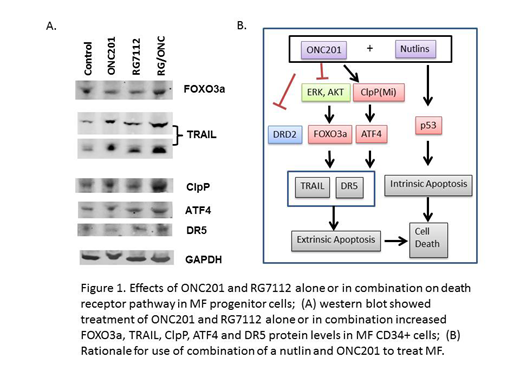
We next tried to understand the mechanism by which combination therapy with RG7112 and ONC201 specifically targeted MF CD34+ cells. We found increased transcript levels of ClpP in untreated MF CD34+ cells as compared to normal CD34+ cells (p=0.03). Exposure of MF CD34+ cells to this drug combination significantly induced apoptosis of MF CD34+ cell (p=0.04), but did not induce apoptosis of normal CD34+ cells. Treatment of MF CD34+ cells with ONC201 alone increased transcript levels of TRAIL and death receptor 5 (DR5), but treatment with RG7112 alone did not have a similar effect. Furthermore, a combination of RG7112 and ONC201 synergistically increased not only TRAIL and DR5 but also ClpP transcript levels to a far greater extent. Treatment with RG7112 and ONC201, however, alone or in combination did not elevate ClpP, DR5 or TRAIL transcripts level in normal CD34+ cells.
Treatment of MF CD34+ cells with either RG7112 or ONC201 alone increased TRAIL and DR5 protein levels to a modest degree but the degree of elevation was far greater with a combination of these two drugs. Treatment with RG7112 and ONC201 alone or in combination also increased FOXO3a, ClpP and ATF4 protein levels which are the upstream regulators of TRAIL and DR5, respectively (Figure 1).
Summary: These data indicate that ONC201 combined with RG7112 is a potentially effective strategy for the treatment of MF. This drug combination selectively targets MF CD34+ cells by activating different apoptotic pathways that are both p53 dependent and p53 independent. Such a therapeutic approach would be anticipated to promote the elimination of both p53 wild type and p53 mutated MPN stem cell clones resulting in a greater depletion of MF but not normal stem cells.
Hoffman:Merus: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal