Bone and bone marrow are not only anatomically, but also functionally interdependent. In a systematic review, we examined bone health in patients with hematopoietic disorders and demonstrated that an increased hematopoietic cell proliferation, such as in patients with hemolytic anemias, was associated with bone loss, while bone marrow hypocellularity, such as in patients with chronic myelofibrosis (CMF), was associated with bone gain [Steer K et al. J Bone Miner Res 2017]. Since bone mass in CMF increases at the expense of bone marrow, it contributes to patients' morbidity as it is associated with bone pain, and mortality as it may lead to bone marrow failure. A mouse model with a global knockout of the megakaryocyte (MK) lineage specific inhibitory receptor G6b-B was shown to develop myelofibrosis secondary to aberrant platelet production and function [Mazharian A et al Sci Signal 2012]. Moreover, a group of patients with primary myelofibrosis was identified to have loss-of-function mutations in the G6b-B gene [Hofmann I et al Blood 2018]. The objective of this study was to characterize temporal changes in the skeleton of the G6b-B knockout mice.
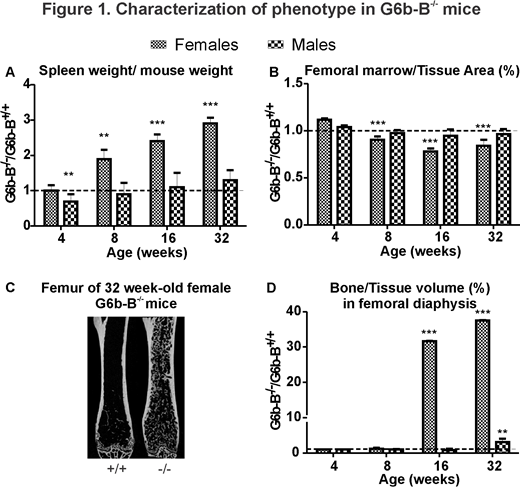
We examined age- and sex-related changes in 4, 8, 16, and 32 week-old G6b-B+/+, G6b-B-/- female and male mice. Starting from 8 weeks-of-age, spleen progressively increased in female G6b-B-/- mice compared to corresponding G6b-B+/+ mice, reaching 2.9-fold increase at 32 weeks (p < 0.001) (Fig.1A). Micro-computed tomography analysis of femur demonstrated that starting at 8 weeks of age female G6b-B-/- mice had a significantly higher proportion of cortical bone and a respectively lower proportion of marrow (Fig.1B). Starting at 16 weeks of age, female G6b-B-/- mice developed trabecula in the medullary cavity normally occupied by the bone marrow, which by 32 weeks led to a 38-fold increase (p < 0.001) in the proportion of bone to tissue volume compared to G6b-B+/+ (Fig.1C,D). At 32-weeks of age, female G6b-B-/- mice also demonstrated a 7-fold increase in BV/TV (p < 0.001) in the region of metaphysis. While some abnormalities were found in male G6b-B-/- mice, they were considerably less severe compared to females. To establish whether the observed bone phenotype is due to MK and platelet functional defects, we performed microcomputed tomography analysis on femurs of 22 week-old G6b-Bfl/fl;Gp1ba-Cre-/- mice with a MK/platelet-specific knockout of G6b-B. Changes in trabecular bone of G6b-Bfl/fl;Gp1ba-Cre-/- mice recapitulated changes of G6b-B-/- mice. However, periosteal perimeter in male G6b-Bfl/fl;Gp1ba-Cre-/- mice was significantly larger, and in female G6b-Bfl/fl;Gp1ba-Cre-/- mice - significantly smaller than in corresponding control mice, while in global G6b-B-/- mice periosteal perimeter was not affected.
Female G6b-B-/- mice demonstrated severe splenomegaly as well as progressive osteosclerosis, which was confirmed in G6b-Bfl/fl;Gp1ba-Cre-/- mice, indicating that trabecular bone gain in G6b-B-/- mice is consequent to a MK disfunction. Dramatic sexual dimorphism suggests that sex-related factors play an important role in the development of osteosclerosis. The differences in cortical bone phenotype between the global and conditional knockout of G6b-B suggest the potential role of G6b-B signaling in osteoclasts or osteoblasts. This study demonstrates that MK-associated myelofibrosis is sufficient to induce osteosclerosis of bone marrow, and that sex hormones play an important role either in protecting male mice from osteosclerosis or in exacerbating osteosclerosis in female mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal