Introduction
Langerhans cell histiocytosis (LCH) is an uncommon histiocytic disorder which is now categorized as a hematopoietic neoplasm. Most treatment and outcomes data in LCH are derived from pediatric studies, and there is a lack of FDA-approved treatment options for adult LCH. There is some evidence that cladribine may be toxic to monocytes and monocyte-derived dendritic cells. In this study, we report the efficacy of cladribine in adult LCH patients seen at our institution.
Methods
We retrospectively reviewed the charts of all LCH patients seen at our institution between 1998 and 2018. Where necessary, the radiological images and histopathological slides were reviewed by an expert radiologist and pathologist. Since prospective uniform response assessment was not performed, we utilized the clinical documentation and radiological reports to assess the overall response rate (ORR). All time to event analyses were performed from the time of cladribine initiation.
Results
We included a total of 37 adult LCH patients in the study. The median age at diagnosis for this cohort was 35 years (range, 21-76), and 51% were males. Although 31 (84%) patients had multi-system disease, all patients had more than one LCH lesion (multifocal). Most commonly involved organs were bone (65%), lung (60%), skin (38%), lymph nodes (30%), and pituitary/hypothalamus (27%). BRAF-mutational analysis was performed in 13 patients, with 7 (54%) demonstrating the presence of BRAF-V600E mutation. Cladribine was administered as first line therapy in 22 (59%) patients and subsequent line treatment in 15 (41%). Of the 15 patients who received cladribine in subsequent line, surgery (n=3), radiation (n=3), steroids (n=3), antibiotic with inhaled steroids (n=1), vinblastine (n=3), topical nitrogen mustard cream (n=1) and vemurafenib (n=1) were the treatments utilized before the initiation of cladribine. Two patients received the drug more than once during the course of their disease. The dosing of cladribine for all patients was based on one of the two intravenous regimens (0.14 mg/kg for days 1-5 every 28 days or 5 mg/m2 for days 1-5 every 28 days).
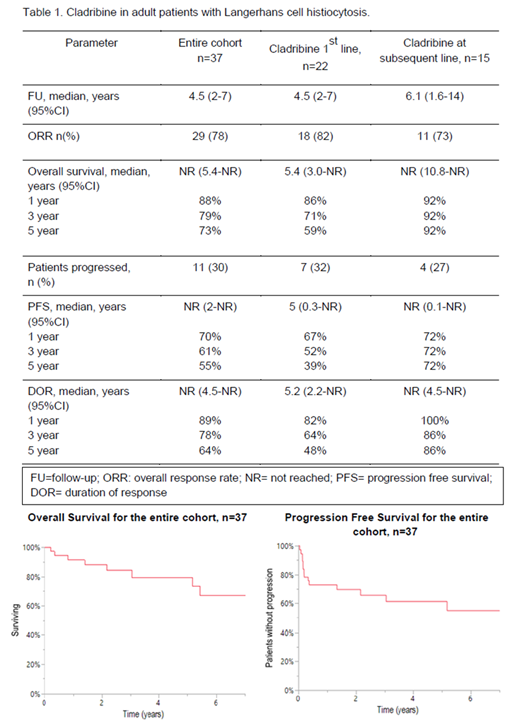
The median follow-up for the entire cohort was 4.5 years (95% CI:2-7) and the treatment outcomes are shown in Table 1. Median number of cycles of cladribine administered was 1.5 (range, 1-9). Clinical/radiographic responses were noted in 29 (78%) patientsORR was 78%, with 24% complete responses and 54% partial responses (PR). Responses were seen in various disease sites: lung nodules/infiltrates (13/29, 45%), bone (12/29, 41%), lymph nodes (8/29, 28%), skin (3/29, 10%), pituitary/hypothalamus (4/29, 14%). Eight (22%) patients did not respond and had progressive disease (PD)- cystic/bullous lung disease (n=2), skin (n=2), abdominal/peritoneal lymph nodes (n=2), and hypothalamus (n=3).The treatment was well tolerated, with grade 3 or above adverse effects seen in three patients: two with lymphopenia requiring dose delays and one with congestive cardiac failure leading to drug discontinuation. After initial disease response, PD was seen in three patients. 89%, 78%, 64% of those who responded initially maintained their responses at years 1, 3, and 5, respectively (Table 1). The 5-year progression free survival (PFS) was 55% for the entire cohort.
BRAF-status was evaluated on 13 of 37 patients in the entire cohort (35%): BRAFV600E positive [n=7 (53%)] and WT [n=6 (46%)]. Of the 7 patients who had BRAFV600E mutation, responses were seen in 71%, while 100% of those without BRAFV600E achieved a response (p=0.09). At the time of last follow-up, 9 patients (24%) were dead. Of those, cause of death were available on 5 patients; due to LCH (n=1), stroke (n=1), gastrointestinal hemorrhage (n=1), acute myeloid leukemia (n=2).
Conclusion
In our study, cladribine monotherapy yielded a high ORR, with the majority of patients achieving a PR. The responses were durable with a small risk of subsequent disease relapse. Responses were seen irrespective of the presence of BRAFV600E mutation. Cladribine was well tolerated overall, and may be considered a potential therapy for adult LCH patients.
Vassallo:Sun Pharmaceuticals: Research Funding; Pfizer: Research Funding; Bristol Myers Squibb: Research Funding; Sun Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding.
Cladribine for langerhans cell histiocytosis
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal