Background: Myeloproliferative neoplasms (MPNs) are chronic myeloid malignancies with markedly heterogeneous disease course, and are associated with underlying inflammatory states that promote development of thrombotic events and acquisition of comorbidities. There is poor understanding of health care resource utilization (HRU) and cost of treatment in patients with MPNs.
Objectives:To estimate and compare the HRU and cost of treatment for MPN patients (Essential Thrombocythemia, ET; Polycythemia Vera, PV; and Myelofibrosis, MF) with matched controls, and investigate the impact of patient characteristics and health service factors on the cost of treatment.
Study design: Retrospective, population-based, matched-cohort study, using provincial health databases of Ontario's single payer universal health system.
Study population: Cases were individuals in the Ontario Cancer Registry, diagnosed with MPN (Total n= 7130; ET, n=3481; PV, n=2618; MF, n=1031), from 2004 to 2016. Controls were individuals in the general population of Ontario, without a diagnosis of MPN. Each case was matched with four controls on age, sex, geographical location, and neighborhood income quintile. Baseline parameters including thrombosis and other comorbidities were collected during two-years prior to the date of MPN diagnosis. The baseline comorbid disease burden was measured using the Aggregated Diagnostic Group (ADG) score with a larger number of ADGs representing a greater comorbid disease burden (https://www.johnshopkinssolutions.com/wp-content/uploads/2014/04/ACG-White-Paper-Applications-Dec-2012.pdf).
Main outcome measures:For each case and its controls, direct medical costs were obtained by costing all health care-related resources and expressed as mean per person year costs ((2018 Canadian Dollars, $1 CDN = $0.76 USD) to adjust for variable length of follow-up. Linear regression analysis was performed to assess the impact of baseline factors on the cost of treatment for MPN and represented as rate ratios (95% CI).
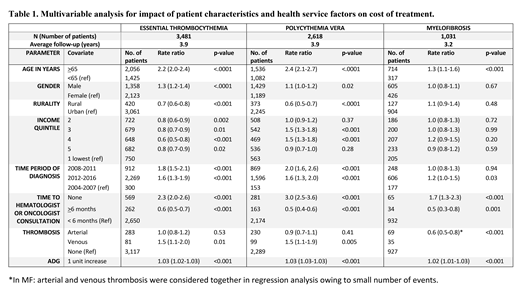
Results:The mean duration of follow-up in years (cases vs controls) was 3.9 vs 4.3 for ET; 3.9 vs 4.2 for PV and 3.2 vs 4.9 for MF. The total follow-up duration was 27449 person years for all MPN cases, and 124963 person years for all controls. Comorbidities (congestive heart failure, chronic obstructive pulmonary disease, coronary artery disease, stroke, chronic renal failure, chronic liver disease, and pre-diagnosis arterial and venous thromboses were significantly higher in cases as compared to controls (p<0.001). Mean (+SD) ADG score (cases vs controls) were 19.2 vs 10.6 for ET, 19.5 vs 10.9 for PV, and 22.7 vs 11.8 for MF (p<0.001 for all three MPN types).
The mean per-patient-year direct medical cost of treatment for patients with ET was $18,840 (2.3 X controls), for PV $18,966 (2.2 X controls) and for MF, $38,147 (4.5 X controls). Factors impacting costs of treatment are summarized in Table 1. In all MPNs, increased health expenditure was associated with older age (>65 years), those who never consulted a specialist in hematology-oncology, and increasing morbidity denoted by ADG score. Patients who were first seen by the specialist >6 months after the diagnosis incurred significantly lesser cost of treatment due to less comorbidity burden as noted by the lower ADG score for patients with >6 months vs <6 months to specialist referral (15 vs 18 for ET, 17 vs 19 for PV and 21 vs 22 for MF). History of venous thrombosis in ET and PV patients predicted higher cost of treatment but not arterial thrombosis due to confounding between arterial thrombosis and higher ADG score.
CONCLUSION: MPN patients have substantial higher direct medical cost of treatment compared to matched-controls and have a high co-morbidity burden at diagnosis that significantly predicted higher cost of treatment. After adjusting for co-morbidity, history of venous thrombosis at diagnosis showed significantly higher cost of treatment in ET and PV. In addition, MPN patients who were never referred to a specialist incurred significantly higher cost of treatment.
Gupta:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Research Funding; Sierra Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal