Introduction: CV events in CP-CML can negatively impact clinical and survival outcomes (Coutinho et al, Clin Lymphoma Myeloma Leuk, 2017), and CV-related hospitalization (hosp.) is costly (Nicholson et al, Clinicoecon Outcomes Res, 2016; Naccarelli et al, Clin. Cardiol, 2010). Mounting evidence suggests the common CV risk factors observed in individuals over the age of 60 combined with tyrosine kinase inhibitor (TKI) therapy may contribute to CV events in patients (pts) with CP-CML (Moslehi & Deininger, J Clin Oncol, 2015; Aghel et al, Vasc Health Risk Manag, 2017). SIMPLICITY is an ongoing observational study of pts with CP-CML receiving first-line (1L) TKIs in routine clinical practice in Europe and the United States (US). Here, we assess CV-related hosp. rates for SIMPLICITY pts in the US receiving 1L imatinib (IM), dasatinib (DAS), or nilotinib (NIL), including the incidence of hospital admissions and length of hospital stay (LOS), and related costs.
Methods: In US pts, CV-related hosp. during 1L IM, DAS, or NIL therapy were identified from review of the electronic case report forms completed by clinicians. To control for possible confounding effects of multiple lines of TKI therapy, pts were followed from 1L TKI initiation to 30-days post-treatment or TKI switch, whichever came first. CV-related hosp. rates and LOS during 1L therapy were evaluated on a per-1,000-pt-year (PY) basis. For each CV event, mean total hosp. costs were derived from the 2016 Healthcare Cost and Utilization Project-National (Nationwide) Inpatient Sample database, to which professional fees from peer-reviewed literature were added; the medical component of the Consumer Price Index was used to convert 2016 costs to 2018 US dollars ($). Statistical comparisons for continuous variables were made using t-tests and the Mann-Whitney U test, and chi-square test for categorical variables. Time to first CV-related hosp. was evaluated using Kaplan-Meier methods with the log-rank test.
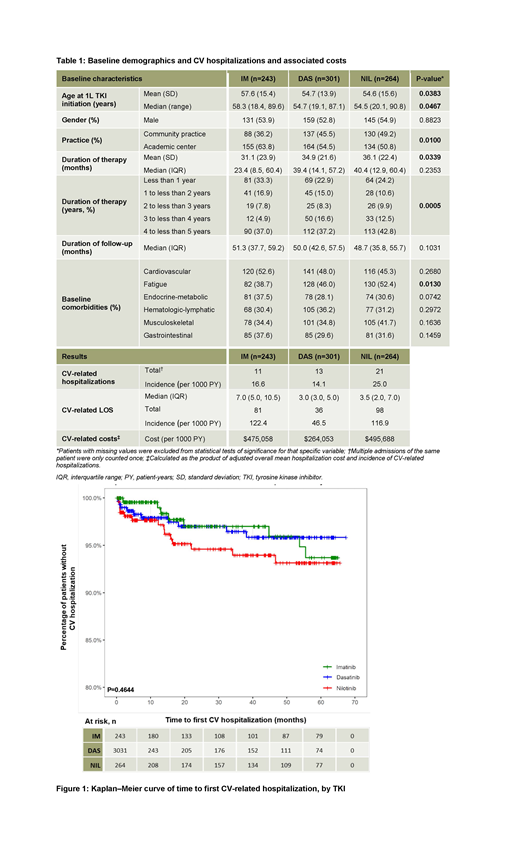
Results: In the US, 808 pts were receiving 1L IM (n=243), DAS (n=301), or NIL (n=264); median follow-up was approximately 4 years (48.7-51.3 months). Age, treatment center type, and baseline fatigue differed significantly between the groups (p<0.05); stratification of duration of therapy in pts showed significant differences between the three TKI cohorts (p=0.0005; Table 1). The number of pts with CV-related hosp. was similar between IM (11 [4.5%]) and DAS (13 [4.3%]) cohorts, but higher in the NIL cohort (21 [8.0%]), equating to 16.6, 14.1, and 25.0 CV-related hosp. per 1,000 PY, respectively. Total CV-related LOS was 81, 36, and 98 days for the IM, DAS, and NIL groups, respectively, translating to 122.4, 46.5, and 116.9 hospital stay days per 1,000 PY. Most frequently experienced CV events were cardiac failure (IM=3.0, DAS=3.2, NIL=7.2 [per 1,000 PY]) and hemorrhage (IM=7.6, DAS=2.2, NIL=4.8 [per 1,000 PY]); rate of the latter was lowest in the DAS group. The rate of myocardial infarction-related hosp. was 1.5 and 2.4 per 1,000 PY for IM and NIL cohorts, respectively. Most inpatient days were due to cardiac failure (IM=13.6, DAS=10.8, NIL=56.1 [per 1,000 PY]) and hemorrhage (IM=80.1, DAS=5.4, NIL=22.7 [per 1,000 PY]); for DAS and NIL, cardiac failure caused the most inpatient days, whereas CV-related hemorrhage was responsible for the most IM-related hospitalization days. 43.8% of the observed CV-related hosp. occurred within 12 months of 1L TKI initiation, with 68.8% occurring by the 18-month mark (Figure 1). No difference in time to first CV-related hosp. with 1L TKI was seen between cohorts (p=0.4644). CV-related hosp. costs were highest among pts receiving IM or NIL, approximately twice that of the DAS cohort. Estimates derived from the product of mean hosp. cost per CV event and the rate of hosp. showed total CV hosp. costs of $475,058, $264,053, and $495,688 per 1,000 PY for IM, DAS, and NIL, respectively.
Conclusions: CV-related events and hosp. were reported among US pts with CP-CML receiving 1L TKIs; the majority of which occurred within 18 months of TKI initiation. The incidence of CV-related hosp. and LOS, and mean hosp. costs were lowest among pts receiving DAS compared with the IM and NIL cohorts. The potential for added morbidity, healthcare utilization, and associated costs due to CV complications must be considered when making decisions on the appropriate TKI for individual pts with CP-CML.
Goldberg:Cancer Outcomes Tracking and Analysis (COTA) Inc.: Equity Ownership; Bristol-Myers Squibb: Consultancy; COTA: Equity Ownership. Mauro:Bristol-Myers Squibb: Consultancy; Novartis Oncology: Consultancy, Research Funding; Pfizer: Consultancy; Takeda: Consultancy. Cortes:Novartis: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; BiolineRx: Consultancy; Takeda: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Keating:Bristol-Myers Squibb: Employment. Bhandari:SmartAnalyst India (Pvt.) Ltd.: Employment, Other: I am an employee of SmartAnalyst India (Pvt.) Ltd., a subsidiary of SmartAnalyst Inc. which was contracted by Bristol-Myers Squibb for carrying out this analysis. Chen:Bristol-Myers Squibb: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal