Introduction: For patients with relapsed or primary refractory (rel/ref) diffuse large B-cell lymphoma (DLBCL) who respond to salvage chemotherapy, high-dose chemotherapy and autologous hematopoietic cell transplantation (HDT-AHCT) is considered standard of care. Patients with refractory disease to salvage chemotherapy, defined as stable disease (SD) or progressive disease (PD), by functional imaging are ineligible for HDT-AHCT, and have a poor prognosis. In practice, we have attempted to salvage these patients with radiation therapy (RT) to residual sites of active disease prior to consolidative HDT-AHCT. The outcome of this unique combined modality salvage paradigm has not been previously reported.
Methods: We retrospectively reviewed all patients with rel/ref DLBCL who received salvage chemotherapy followed by salvage RT and HDT-AHCT between the years of 2000 and 2017 at a single center. Only patients with SD or PD as defined on the 5-point Deauville scale after salvage chemotherapy and who had at least 1 year of follow-up were included in this analysis. The second-line age-adjusted International Prognostic Index (sAAIPI) was determined at the time of initiation of salvage chemotherapy.Survival functions were estimated by the Kaplan-Meier method and compared using a log-rank test.
Results: Thirty-six patients, 12 with relapsed and 24 with primary refractory disease, with a median age of 44 years (range: 19-68 years) were analyzed. Twenty-three patients had DLBCL while 13 had primary mediastinal B-cell lymphoma (PMBCL). The majority of patients had KPS 80-100 (n=32, 89%), 0-1 extranodal sites (n=30, 83%), and normal LDH (n=21, 58%). The sAAIPI scores for this cohort were as follows: 0 (n=10), 1 (n=21), 2 (n=4), and 3 (n=1). All patients received salvage chemotherapy with subsequent functional imaging showing SD (n=32) and PD (n=4) and then went on to receive salvage RT to the sites of active disease. Median RT dose was 39.6Gy (range: 30-54Gy). Six patients also received TBI as part of their conditioning regimen prior to HDT-AHCT.
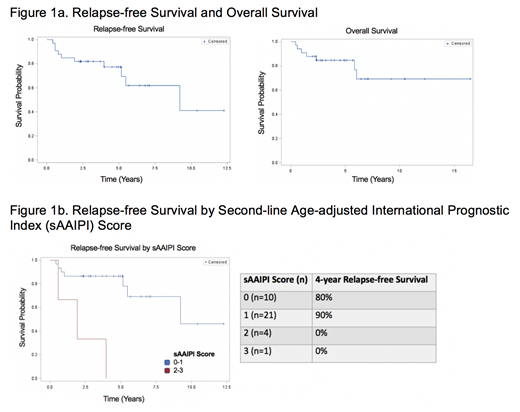
With median follow up of 4.0 years (range: 1.0-12.3 years) for survivors, 4-year relapse-free survival (RFS) was 75.6% and 4-year overall survival (OS) was 80.3% (Figure 1a). There was no significant difference in 4-year RFS for patients with relapsed versus primary refractory disease (80.2% vs 74.8%, p=0.59). PMBCL patients had better RFS than DLBCL patients (92.3% vs 67.8%, p=0.12).
Using the composite sAAIPI score was highly prognostic with worse outcomes for patients with higher risk sAAIPI scores. By sAAIPI score, 4-year RFS was 80.0% for a score of 0, 90.2% for a score of 1, and 0% for scores of 2 and 3. Patients with low- and low-intermediate risk sAAIPI scores of 0 and 1 had improved RFS as compared to patients with sAAIPI scores of 2 and 3 (87.0% vs 0%, p<0.0001 (Figure 1b).
Conclusions: Patients with chemorefractory rel/ref DLBCL who have had minimal or no response to systemic salvage therapy may benefit from salvage RT to the residual PET-avid disease followed by HDT-AHCT, particularly if their sAAIPI score is ≤ 1. The outcome of this retrospective cohort is markedly superior to outcomes described in the literature for this high-risk population and represents a promising treatment paradigm to be further explored. Emerging data suggest similar patients may benefit from CAR T-cell therapy. Given the limited availability and high cost of CAR T-cell therapy, we suggest there may be a role for sequencing this combined-modality salvage paradigm prior to CAR T-cell therapy in order to provide these poor-risk patients with an additional line of therapy.
Scordo:Angiocrine Bioscience, Inc.: Consultancy; McKinsey & Company: Consultancy. Sauter:Sanofi-Genzyme: Consultancy, Research Funding; GSK: Consultancy; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Celgene: Consultancy; Juno Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal