Background:
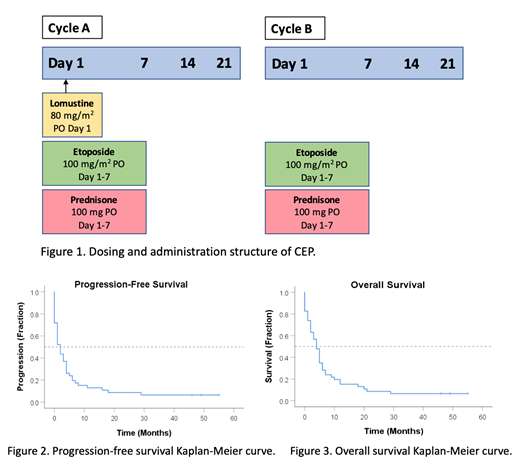
There is no current standard of care therapy for the management of patients with aggressive lymphomas in the relapsed or refractory (R/R) setting who are not candidates for intensive salvage chemotherapy and/or autologous stem cell transplant (ASCT). The combination of lomustine (CCNU), etoposide and prednisone (CEP) is an oral chemotherapeutic regimen that is modified from CCEP used in Hodgkin Lymphoma (CCNU, chlorambucil, etoposide and prednisone). CEP consists of alternating A & B cycles as follows (Figure 1): 'A' cycles contain lomustine 80 mg/m2 on day 1 as well as etoposide 100 mg/m2 and prednisone 100 mg on days 1-7. 'B' cycles contain only the etoposide and prednisone. Cycles are given every 21 days apart. To date there has been no published data on CEP's efficacy and tolerability in aggressive R/R lymphoma. In this study, we describe our institutional experience with CEP at the London Regional Cancer Program (LRCP) in London, Ontario for patients with R/R aggressive lymphomas unable to tolerate intensive chemotherapy.
Methods:
We conducted a retrospective review of patients who received CEP at LRCP between January 2014 and May 2018 for R/R aggressive lymphomas. The primary endpoint was overall response rate (ORR). Secondary endpoints included overall survival (OS), progression-free survival (PFS), and adverse effects related to CEP. Kaplan-Meier survival curves modeled PFS and OS. We also performed univariate and multivariate analyses to assess whether pre-specified variables (age, IPI score at diagnosis, LDH at time of treatment, and the number of prior lines of therapy) were independently associated with response to CEP (using linear regression) or progression-free survival (using the Cox proportional hazards model). IPI score (0-2 vs. 3-5) and the number of prior lines (1-2 vs. ≥ 3) were analyzed as binary variables due to the overall sample size.
Results:
46 patients received CEP at LRCP during the study period. Diffuse large B-cell lymphoma was the most common diagnosis (70%) with Hodgkin lymphoma (6.5%) and peripheral T-cell lymphoma (6.5%) being the next most common. The median age of patients starting CEP was 76, with the median number of prior therapies being 2 (range 1-6). The primary outcome was ORR, which was 41% in our population, with a median time to response of 23 days. The median PFS was 2 months while the median OS was 4 months (Figure 2). The 2-year PFS and OS were both 8.7%. The median CEP treatment period was 1.5 full cycles while the longest CEP treatment period was 21 consecutive cycles. Univariate analysis revealed that lower IPI score (p=0.037) was significantly associated with ORR, while lower LDH (p=0.029) was significantly associated with PFS. Multivariate analyses revealed no factors associated with ORR, while lower LDH (p=0.017) and lower IPI (p=0.037) were associated with PFS. Toxicities were manageable with 48% with cytopenias of any grade and the most common grade 3-4 toxicity was febrile neutropenia in 13%.
Conclusions:
CEP is a safe, and convenient, all-oral palliative regimen for R/R aggressive lymphoma who are not eligible for intensive salvage chemotherapy or ASCT. The median OS in our population is comparable to pooled clinical trial and academic center data from non-transplant eligible patients with R/R DLBCL (Crump et al, Blood, 2017). Some patients even in spite of low performance statuses were able to tolerate many cycles of CEP with a long period of disease control, suggesting it is a reasonable treatment option for frail patients to balance disease control with an acceptable side-effect profile.
Lam:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Other: Education Grant; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Phua:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Teva: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda/Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; NovoNordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bioverativ: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal