Introduction: Multiple case reports have described lymphomas and plasma cell neoplasms developing after trauma 1-4. It has been hypothesized that trauma-induced inflammation may contribute to the neoplasia, and that the site of trauma may serve as the nidus (locus minoris resistentiae) 1, 5. However, to our knowledge there are no studies demonstrating causality. Traumatic injuries sustained during combat have been linked with higher rates of hypertension, coronary artery disease, diabetes mellitus and chronic kidney disease 6. Whether severe injury also increases the risk of hematologic malignancies is unknown. We hypothesized that combat injured veterans have higher rates of lymphoma and plasma cell dyscrasias than un-injured combat veterans.
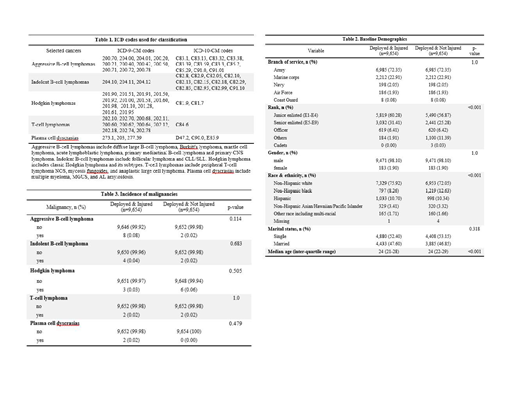
Methods: This was a retrospective cohort study of US military personnel injured during combat operations in Iraq or Afghanistan from 2002-2015 extracted from the Department of Defense (DoD) Trauma Registry. Patients were excluded if they died in theater, had multiple battle injuries, had pre-existing cancer diagnoses or missing data. A comparator arm of deployed and un-injured Iraq and Afghanistan combat veterans was obtained from the Military Health System Data Repository (MDR) matched for year of birth, branch of service, and sex. Cancer diagnoses were defined using International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) Clinical Modification codes obtained from both the DoD and the Veterans Health Administration through the MDR and the Veterans Informatics and Computing Infrastructure, respectively. Malignancy classifications by ICD codes are demonstrated in Table 1 and broken down into aggressive B-cell lymphomas, indolent B-cell lymphomas, Hodgkin lymphoma, T-cell lymphomas and plasma cell dyscrasias. Statistical analyses were performed with the chi-square test and fisher's exact test. This study was approved by the IRB at David Grant USAF Medical Center.
Results: There were 9,654 subjects each in the injured and un-injured cohorts. Baseline demographics are demonstrated in Table 2. The mean age was 26 and most patients were junior enlisted, members of the Army, males, and of non-Hispanic white ethnicity/race. The average post-trauma follow-up time was 5.3 years. Overall rates of aggressive B-cell lymphomas, indolent B-cell lymphomas, Hodgkin lymphoma, T-cell lymphomas and plasma cell dyscrasias were low, as demonstrated in Table 3, and there were no statistically significant differences in incidence rates between the two cohorts.
Discussion: Traumatic injuries can activate innate immune responses involving inflammatory cytokines, complement, coagulation and dendritic cells, resulting in a pro-inflammatory state 7. Preclinical studies reveal that such inflammation may hamper T-cell immune-surveillance needed to eradicate malignancy 8. However, in our cohort of severely injured combat veterans, rates of lymphomas and plasma cell dyscrasias were not increased over un-injured combat veterans. Strengths of this study include a well-characterized cohort with a matched comparator arm enabling vigorous examination of the impacts of traumatic injuries on cancer. Limitations include retrospective design and relatively short follow up. It is possible that differences will emerge with longer follow up.
Conclusions: Despite critical combat trauma injuries being associated with a heightened risk of multiple chronic comorbidities, they do not appear to heighten the risk of lymphoid or plasma cell neoplasms within the first 5-years.
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the U.S. Air Force, David Grant USAF Medical Center, the Uniformed Services University or the Department of Defense.
References:
1. Kriwalsky MS, Oral Surg, 2010.
2. Huang J, Medicine, 2019.
3. Stemberga V, Hematol Oncol, 2003.
4. Erdogan B, Eur Spine J, 2005.
5. Lo Schiavo A, Clin Dermatol, 2014.
6. Stewart IJ, Circulation, 2015.
7. Huber-Lang M, Nature Immunology, 2018.
8. Krall J, Science, 2018.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal