Introduction: Previous studies have highlighted the potential of cell-free DNA (cfDNA) to assess the mutational profile and copy number alterations (CNA) in diffuse large B-cell lymphoma (DLBCL), usually performed in tissue biopsies, both at diagnosis and relapse. In addition, the quantitative levels of cfDNA might be a surrogate of the lymphoma tumor burden and could therefore predict response to therapy, progression-free survival (PFS) and overall survival (OS). The aim of this study was to analyze the mutational profile and CNA at diagnosis using cfDNA, to compare these results with those obtained from formalin-fixed paraffin-embedded (FFPE) biopsies, and to estimate the correlation of pre-treatment levels of cfDNA with FDG-PET/CT Total Metabolic Tumor Volume (TMTV) and their impact in the outcome of DLBCL patients.
Methods: We included 79 patients (41M/38F , median age 63 years) diagnosed with DLBCL according to the WHO criteria in a single institution between 2016 and 2018. All patients received chemoimmunotherapy. After frontline treatment, 56 patients achieved a complete (CR) response, 4 partial response, 16 were refractory, including 7 early deaths, and in 3 cases the response was not yet evaluable. Samples were obtained at diagnosis before starting treatment. cfDNA extraction was performed from 2-4 mL of plasma collected in PAXgene Blood ccfDNA tubes (Qiagen) and size distribution of DNA fragments was analyzed using the Agilent 2100 Bioanalyzer. Tumor genomic DNA (gDNA) was isolated from FFPE diagnostic tissue biopsies. A panel of 115 genes was enriched using a hybridization capture-based protocol from 10-30 ng of cfDNA and 150 ng of gDNA (ThruPlex Tag-seq kit and SureSelectXT enrichment reagents, Agilent Technologies) and sequenced in a MiSeq instrument (Illumina). CNA were examined using CNVkit software toolkit. cfDNA levels were reported as haploid genome equivalents per mL of plasma and expressed as a base 10 logarithm (log hGE/mL). Quantitative analysis of TMTV was performed using the semiautomatic MIM software, with a fixed SUV>2.5 thresholding method for segmentation.
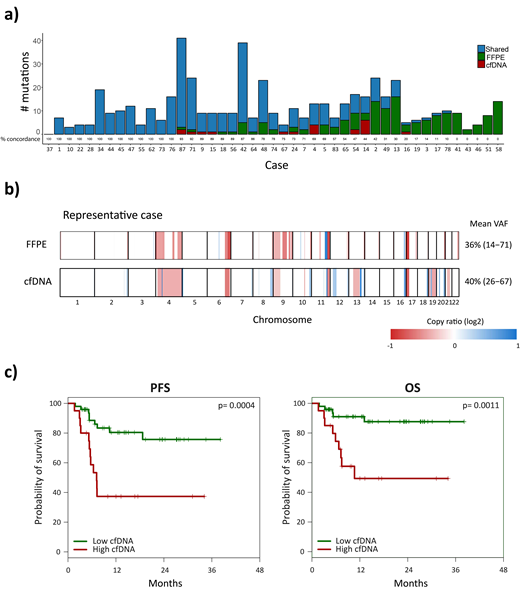
Results: A median of 15.6 ng/mL (range: 4-754 ng/mL) of cfDNA was obtained. At least one mutation could be detected in 69/79 cases (87.3%). The median number of mutations per sample was 6 (range: 0-41 mutations). The genes most frequently mutated at diagnosis in plasma samples were KMT2D, TP53, TNFRSF14, MYD88, BCL2, SOCS1, CREBBP, MYC and EP300. In 45 cases, paired FFPE samples were available. Sensitivity of cfDNA to detect mutations in baseline FFPE samples was 69% (95%CI: 64.1-73.9). In 28 of the 45 cases, >70% of the mutations were observed both in the cfDNA and FFPE samples. In the remaining 17 cases, the number of mutations identified in cfDNA was lower than the one observed in the FFPE sample (Figure 1a). Of note, most of the cases in which mutations were not detected in the cfDNA corresponded to localized stages (11/17, 65%) and/or primary extranodal DLBCL (12/17, 71%). The CNA profile in cfDNA was similar to that reported in tissue, with recurrent deletions of TNSFRS14 (11%), TNFAIP3 (14%), CDKN2A (7%), or TP53 (7%), as well as gains of REL (7%) or BCL2 (5%), among others (Figure 1b).
Median pre-treatment TMTV (N=48) was 292 cm3 (0-4,171 cm3). Higher TMTV predicted for a poorer PFS (HR 4.85; p=0.005). cfDNA concentration significantly correlated with TMTV (R=0.546, p<0.001), confirming that cfDNA measurements are related to the tumor burden of the lymphoma. In addition, patients with high pretreatment cfDNA levels (>3 log hGE/mL) had a significantly lower CR rate, PFS, and OS than those with low levels (CR rate, 47% vs. 92%, p<0.001; 24-month PFS 37 vs. 76%, p=0.0004; and 24-month OS 50 vs. 88%, p=0.001) (Figure 1c).
Conclusions: cfDNA represents a valuable tool to easily assess the genomic landscape and tumor burden in DLBCL, as well as to predict response to therapy and outcome in these patients.
Gine:Janssen: Other: Travel expenses, Research Funding; Roche: Other: Travel expenses, Research Funding; Gilead: Other: Travel expenses, Research Funding. Lopez-Guillermo:Gilead: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal