Introduction
MCL is a rare cancer, and current clinical practice guidelines list multiple reasonable treatment options for MCL. It can be challenging to select treatment (Tx) that optimizes outcomes for patients with a mixture of presenting features. In 2014, we developed an online MCL decision support tool designed to provide expert guidance on optimal treatment for defined patient scenarios. An analysis of the 2014 tool showed that Tx recommendations of community healthcare providers (HCPs) did not consistently align with experts for many patients with MCL (Gopalsamy, et al. Leuk Lymphoma. 2019 Mar 8:1-9). Here we report data from an updated version (2018) of this tool that captures evolving practice patterns among experts and community HCPs and the differences between these 2 groups.
Methods
In September 2018, a panel of 5 experts provided Tx recommendations for 240 patient scenarios in newly diagnosed or relapsed/refractory MCL. These distinct scenarios were defined by patient and disease characteristics that the experts considered important when making treatment choices, including disease setting, patient age and fitness, cytogenetic information, LDH and Ki67 levels, and information on any previous lines of therapy. To use the tool, HCPs entered specific patient/disease characteristics using expert-defined options along with the Tx planned for each case. The users were then shown the 5 MCL expert recommendations and were asked to indicate the impact of the expert recommendations on their planned Tx approach.
Results
A total of 387 HCPs entered 654 patient scenarios in the 2018 online tool. For HCPs who answered an optional question on how many patients with MCL they treat per year (n = 145), 81% indicated that they treated 10 or fewer patients with MCL per year, highlighting the rarity of this disease and lack of experience among many HCPs. Furthermore, 74% of these HCPs indicated that they refer < 50% of their patients with MCL for clinical trials or to academic institutions for treatment.
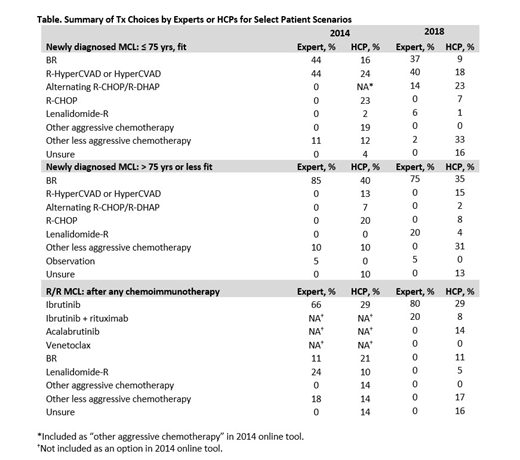
Among experts, the primary consensus for initial Tx recommendations for younger, fit patients with MCL were bendamustine/rituximab (BR) and R-HyperCVAD in both 2014 and 2018. In comparison, HCPs chose these 2 regimens for a minority of these patients with newly diagnosed MCL in both editions of the tool. For older or unfit patients, experts predominantly recommended BR (85% in 2014 and 75% in 2018), whereas only 35% to 40% of HCPs would choose this regimen in this setting (Table). Of note, nearly 20% of the HCPs indicated that they would recommend a very aggressive chemotherapy regimen for older or unfit patients. For patients with disease progression after chemoimmunotherapy, the recommendation of ibrutinib-based therapy increased among experts from 2014 to 2018 from 66% to 100%. By contrast, the percentage of HCPs using ibrutinib-based therapy remained low at 29% or 37% in the 2014 and 2018 tools, respectively. Another 14% of HCPs selected acalabrutinib, another approved BTK inhibitor, in 2018. In addition, approximately 20% of HCPs in both 2014 and 2018 were unsure of the optimal use of additional therapy (eg, ASCT or maintenance therapy) for their patients.
Among users who answered an optional question on the tool's clinical impact, 47% indicated the expert recommendations changed their planned Tx and 38% indicated that expert recommendations confirmed their approach.
Conclusions
The analysis of data from this online tool found several areas of expert consensus regarding Tx for patients with MCL, particularly for patients who are progressing after initial chemoimmunotherapy. However, practice patterns of community HCPs did not align with expert recommendations for the majority of cases entered into the tool. The consistent Tx gap between practice patterns of community HCPs and expert recommendations over this time period suggests a continued need for online decision tools like this and other innovative approaches to involve experts in the care of patients with MCL.
A detailed comparison of expert and user data from the online tool will be presented.
Ansell:LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Trillium: Research Funding; Regeneron: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Regeneron: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment; Regeneron: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Mayo Clinic Rochester: Employment; Bristol-Myers Squibb: Research Funding; Regeneron: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding. Leonard:Nordic Nanovector: Consultancy; Karyopharm Therapeutics: Consultancy; BeiGene: Consultancy; Sutro Biopharma: Consultancy; Gilead: Consultancy; MorphoSys: Consultancy; Merck: Consultancy; Nordic Nanovector: Consultancy; Akcea Therapeutics: Consultancy; MorphoSys: Consultancy; Miltenyi: Consultancy; Epizyme, Inc: Consultancy; AstraZeneca: Consultancy; Bayer Corporation: Consultancy; Celgene: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy; Gilead: Consultancy; Bayer Corporation: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy; Celgene: Consultancy; Karyopharm Therapeutics: Consultancy; Sutro Biopharma: Consultancy; Epizyme, Inc: Consultancy; Merck: Consultancy; Sandoz: Consultancy; Akcea Therapeutics: Consultancy; Miltenyi: Consultancy; Sandoz: Consultancy; ADC Therapeutics: Consultancy; BeiGene: Consultancy. Vose:Acerta Pharma: Honoraria, Other: Grants, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Celgene Corporation: Research Funding; Incyte Corporation: Research Funding; Kite Pharma: Honoraria, Other: Grants, Research Funding; Novartis: Research Funding; Seattle Genetics: Research Funding; AbbVie: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Legend Pharmaceuticals: Honoraria. Wang:BioInvent: Consultancy, Research Funding; Dava Oncology: Honoraria; Aviara: Research Funding; Juno Therapeutics: Research Funding; Celgene: Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; VelosBio: Research Funding; Acerta Pharma: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Guidepoint Global: Consultancy; MoreHealth: Consultancy, Equity Ownership; Loxo Oncology: Research Funding. Flowers:BeiGene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Millenium/Takeda: Research Funding; V Foundation: Research Funding; Bayer: Consultancy; Acerta: Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; TG Therapeutics: Research Funding; Burroughs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; Optimum Rx: Consultancy; Spectrum: Consultancy; Pharmacyclics/Janssen: Consultancy, Research Funding; AstraZeneca: Consultancy; Gilead: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Karyopharm: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal