Background: Cluster of differentiation 47 (CD47), a widely expressed cell-surface ligand,is overexpressed in various malignancies and is correlated with worse outcomes in NHL. Interaction of CD47 and signal regulatory protein-α (SIRPα) delivers an antiphagocytic 'don't eat me' signal to promote tumor cell evasion from macrophages. CC-90002 is a humanized IgG4-PE CD47 antibody that inhibits CD47-SIRPα interaction and enabled phagocytosis across a panel of cancer cell lines (Narla et al. AACR.2017). In addition to high binding affinity, CC-90002 is potentially differentiated from other CD47 immunotherapies by its lack of ability to induce hemagglutination of red blood cells or hemolysis in nonclinical studies. CD47 antibodies can synergize with the CD20 antibody rituximab to induce phagocytosis of NHL cells in vitroand to eliminate lymphoma in mouse models (Chao et al. Cell.2010). We therefore examined CC-90002 plus rituximab for treatment of R/R NHL.
Methods: This 2-part, phase I, multicenter study (CC-90002-ST-001; NCT02367196) is evaluating CC-90002 in subjects with advanced solid and hematologic malignancies. Part B of the study (reported here) is examining CC-90002 in combination with rituximab in subjects with CD20-positive R/R NHL. Dose escalation followed a modified 3+3 design. Subjects received escalating doses of CC-90002 intravenously at 4, 8, 15, 20, or 30 mg/kg every 2 weeks (Q2W) on days 1 and 15 plus rituximab 375 mg/m2 given on days −15, −8, and −1 and day 8 of cycles 1-6, 8, 10, and 12 in 28-day cycles. Primary endpoints are dose-limiting toxicities (DLTs), nontolerated dose (NTD), and maximimum tolerated dose. Secondary endpoints are pharmacokinetics, preliminary efficacy per International Working Group Response Criteria for NHL (Cheson et al. J Clin Oncol.2014), and frequency of antidrug antibodies.
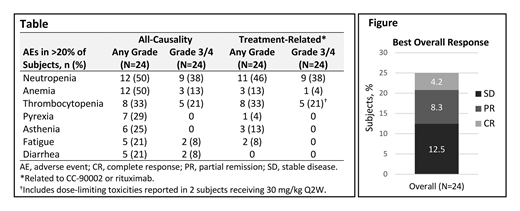
Results: Overall, 28 subjects have been enrolled and 24 were treated. As of July 5, 2019, 20 subjects had discontinued the study, most commonly for progressive disease (PD; n=9) or death (n=7). The median age at enrollment was 64 years (range, 27−81), and subjects had received a median of 3 prior systemic therapies (range, 2−9). Subjects received a median of 2 cycles of CC-90002 plus rituximab (range, 1−18). There were no CC-90002 dose reductions but 7 subjects had their dose interrupted, mostly because of thrombocytopenia and neutropenia. The NTD was established as 30 mg/kg Q2W.DLTs occurred in 3 subjects; 1 subject developed dyspnea attributed to an infusion-related reaction at 15 mg/kg Q2W CC-90002 and 2 subjects had grade 3 thrombocytopenia requiring platelet transfusion occurring at 30 mg/kg Q2W CC-90002. The most common adverse events (AEs) were hematologic (Table). Although anemia was common, there was no evidence of hemolysis. The most frequent grade 3/4 AEs were neutropenia (38%) and thrombocytoenia (21%). Seven deaths occurred on study or in follow-up, 6 from PD or complications related to NHL and 1 due to an AE (cytokine release syndrome in a subject who discontinued CC-90002 for PD and enrolled in another trial within the follow-up period). There were no treatment-related deaths. The overall response rate was 13% (95% CI, 2.7−32.4) and the disease control rate was 25% (95% CI, 9.8−46.7; Figure). One subject achieved a durable complete response (8 mg/kg; ongoing in cycle 18) and 2 had partial responses (15 mg/kg and 20 mg/kg); 3 subjects had stable disease. Among responders, the median duration of response was 3.9 months (95% CI, 1.9−3.9). CC-90002 exhibited linear pharmacokinetics at doses ≥15 mg/kg, suggesting target saturation at 15 mg/kg. In addition, a longer half-life was observed at higher (≥15 mg/kg) versus lower doses (t1/2≈ 3−7 days vs 1 day).
Conclusions: The CD47-SIRPα checkpoint inhibitor, CC-90002,plus rituximab demonstrated tolerability and modest clinical activity in this early-phase study of heavily pretreated R/R NHL subjects. AEs were predominantly grade 1/2; cytopenias were the most common AEs with dose-limiting thrombocytopenia observed at 30 mg/kg Q2W. In contrast to other CD47-targeting immunotherapies and consistent with results of preclinical studies of CC-90002, hemolysis at a starting dose of up to 30 mg/kg CC-90002 was not observed. These preliminary data support further evaluation of targeting the CD47-SIRPα pathway in combination with rituximab in NHL.
Abrisqueta:Abbvie: Consultancy, Honoraria, Other: Travel, Accommodations, expenses, Speakers Bureau; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, expenses, Speakers Bureau; Roche: Consultancy, Honoraria, Other: Travel, Accommodations, expenses, Speakers Bureau. Sancho:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Other: Advisory board; Novartis: Honoraria; Kern Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Celltrion: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squib: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cordoba:Janssen: Consultancy, Honoraria, Speakers Bureau; Servier: Consultancy, Honoraria, Speakers Bureau; Kyowa-Kirin: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Roche: Honoraria, Speakers Bureau; FUNDACION JIMENEZ DIAZ UNIVERSITY HOSPITAL: Employment; Celgene: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy. Persky:Sandoz: Consultancy; Morphosys: Other: Member, Independent Data Monitoring Committee; Debiopharm: Other: Member, Independent Data Monitoring Committee; Bayer: Consultancy. Andreadis:Juno: Research Funding; Pharmacyclics: Research Funding; Roche: Equity Ownership; Novartis: Research Funding; Jazz Pharmaceuticals: Consultancy; Celgene: Research Funding; Kite: Consultancy; Gilead: Consultancy; Merck: Research Funding; Genentech: Consultancy, Employment. Huntington:Pharmacyclics: Honoraria; Genentech: Consultancy; Bayer: Consultancy, Honoraria; DTRM Biopharm: Research Funding; AbbVie: Consultancy; Celgene: Consultancy, Research Funding. Carpio:University Hospital Vall D'Hebron: Employment. Morillo Giles:Hospital Universitario Fundacion Jimenez Diaz: Honoraria. Wei:Celgene Corp.: Employment, Equity Ownership. Li:Celgene Corp.: Employment, Equity Ownership. Zuraek:Celgene Corp.: Employment, Equity Ownership, Other: Travel, Accommodations, Expenses. Burgess:University of California: Other: Volunteer clinical faculty, without salary, Patents & Royalties: Patent - T315A and F317I mutations of BCR-ABL kinase domain; Celgene Corporation: Employment, Equity Ownership, Patents & Royalties: Patent - CD47 antibodies and methods of use thereof. Hege:Celgene Corporation: Employment, Equity Ownership, Patents & Royalties; Arcus Biosciences: Membership on an entity's Board of Directors or advisory committees; Mersana Therapuetics: Membership on an entity's Board of Directors or advisory committees; Society for Immunotherapy of Cancer: Membership on an entity's Board of Directors or advisory committees. Martín:iQone: Consultancy; Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel Expenses; Celgene: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Kiowa Kirin: Consultancy; Servier: Honoraria, Other: Travel Expenses; Teva: Research Funding; Janssen: Honoraria, Other: Travel Expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal