Background: Diffuse large B-cell lymphomas (DLBCL) are a heterogeneous group of aggressive B-cell non-Hodgkin lymphomas (NHL). Primary mediastinal B-cell lymphoma (PMBCL) is often grouped and managed as DLBCL, yet PMBCL is biologically and clinically different. Although about two-third of patients are cured with initial therapy, those who are refractory or progress within the first two years do poorly and will die from their disease. There is an unmet need for effective therapeutic approaches in this patient population. Evasion of the host immune responses is an important mechanism for inducing resistance to cancer therapy. Strategies to block molecular and cellular mediators of cancer induced immunosuppression such as programmed death -1 receptor (PD-1) have been explored. Targeting PD-1 immune checkpoints has the potential to play a major role in cancer therapy by reversing tumor immune escape. Trials using PD-1 blockers in advanced hematological malignancies demonstrated remarkable activity in Hodgkin lymphoma. Unfortunately, results in the DLBCL cohort with nivolumab were marginal and short lived. A phase 1b trial in heavily pre-treated relapsed/refractory (RR) PMBCL using pembrolizumab (a PD-1 blocker) showed that it was safe and active with an ORR of 41%. In recent years, several signaling pathways implicated in DLBCL pathogenesis have been targeted, including the PI3K-AKT-mTOR pathway. Copanlisib, a pan-PI3K inhibitor, with particularly potent PI3K-α/PI3K-δ inhibition, showed activity in both indolent and aggressive NHL, with a safer toxicity profile than the FDA-approved agent idelalisib. In a preclinical DLBCL mouse model, treatment with copanlisib resulted in effective down regulation of tumor‐infiltrating T-regulatory cells (Tregs). In the same model, copanlisib, when combined with a surrogate anti‐mouse PD‐1, showed in vivo responses in 75% vs 0% in the monotherapy groups. These data support the rational for the combination of copanlisib and checkpoint blockade in DLBCL.
We developed a phase 2 prospective study with two RR cohorts (DLBCL and PMBCL), treated with the combination of copanlisib and nivolumab.
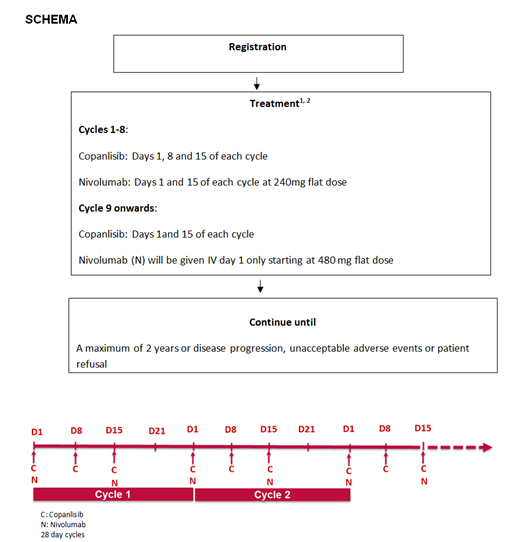
Study Design: copanlisib hydrochloride 60 mg is given IV on days 1, 8 and 15 of cycles 1-8 and days 1 and 15 of subsequent cycles. Nivolumab 240 mg IV is given on days 1 and 15 of cycles 1-8 and 480 mg on day 1 of subsequent cycles. Cycles repeat every 28 days for up to 2 years in the absence of disease progression or unacceptable toxicity. Copanlisib dose will be determined using a safety cohort in 6 patients.
The primary outcome is to assess ORR defined as complete and partial responses (CR+PR). Secondary outcomes are to evaluate safety of the combination; to assess progression free survival (PFS), duration of response (DOR), CR rate, and overall survival (OS). Exploratory objectives are to characterize the effects of the copanlisib and nivolumab combination regimen on tumor cells, tumor microenvironment and the immune response in RR DLBCL and PMBCL. We will also do Lymph3CX assay as an integrated biomarker to distinguish between DLBCL and PMBCL.
Key inclusion criteria: adult patients with RR DLBCL and PMBCL who have measurable disease with at least one lesion that is >15mm in the longest diameter on cross-sectional imaging; After failure of ASCT or after failure of frontline therapy in subjects who declined or are not ASCT candidates; and have appropriate organ function. Key exclusion criteria: High grade B-cell lymphomas; Active, known or suspected autoimmune disease or patients on any prohibited therapies; History of active CNS involvement or leptomeningeal involvement
This study is expected to enroll a maximum of 96 (6 safety cohort, 44 DLBCL and 46 PMBCL) evaluable patients. We anticipate accruing 10 additional patients to account for ineligibility, cancellation, or other reasons. Therefore, the study is expected to accrue a maximum of 106 patients. The largest success proportion where the proposed treatment regimen would be considered ineffective in DLBCL is 25% and 30% in PMBCL. The smallest success proportion that would warrant subsequent studies with the proposed regimen is 45% in DLBCL and 50% in PMBCL. This design has a 90% power with a 1-sided 10% level test.
This study is conducted through NCI-CTEP (NCI Protocol #10193; NCT03484819) and is funded by Bayer. The study is to open in fall of 2019 at 11 ECTCN sites, and is available to interested sites to join in.
Bennani:Kite Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Purdue Pharma: Other: Advisory board; Adicet Bio: Other: Advisory board; Adicet Bio: Other: Advisory board; Purdue Pharma: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Kite Pharma: Other: Advisory board; Kite Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Adicet Bio: Other: Advisory board. Nowakowski:Celgene: Consultancy, Research Funding; Selvita: Membership on an entity's Board of Directors or advisory committees; NanoString: Research Funding; MorphoSys: Consultancy, Research Funding; Genentech, Inc.: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Curis: Research Funding; Bayer: Consultancy, Research Funding. Rimsza:NanoSting: Patents & Royalties: Named inventor on a patent licensed to NanoSting [Institution]. Ansell:Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Regeneron: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Trillium: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Regeneron: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Affimed: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal