Background: CD19 is broadly and homogeneously expressed across different B-cell malignancies and represents an attractive target antigen in patients with B-cell non-Hodgkin's lymphoma (NHL). Tafasitamab (MOR208) is an Fc-enhanced, humanized, anti-CD19 monoclonal antibody. This ongoing study is investigating the single agent antitumor activity in adult patients with relapsed or refractory (r/r) NHL who had received at least one prior rituximab-containing therapy.
Patients and Methods: The study enrolled 92 r/r NHL patients: diffuse large B-cell lymphoma (DLBCL; n=35), mantle cell lymphoma (MCL; n=12), follicular lymphoma (FL; n=34), or other indolent NHL (iNHL; n=11). The median number of prior systemic therapies was three (range 1-15) for the entire patient population. The primary efficacy endpoint was investigator-assessed overall response rate (ORR) based on the revised International Working Group Response Criteria (Cheson et al., et al. J Clin Oncol 2007). Secondary objectives were to evaluate the time-to-response, duration of response (DoR), time to progression and progression-free survival (PFS), and to establish the safety and tolerability of tafasitamab. Patients received up to three 28-day cycles with weekly infusions of 12 mg/kg body weight of tafasitamab. Premedication, including antipyretics, histamine H1 receptor blockers and glucocorticosteroids, was administered for the first three infusions. Patients with ongoing at least partial remission (PR) at the end of Cycle 3 received further tafasitamab treatment until disease progression, either monthly or every second week.
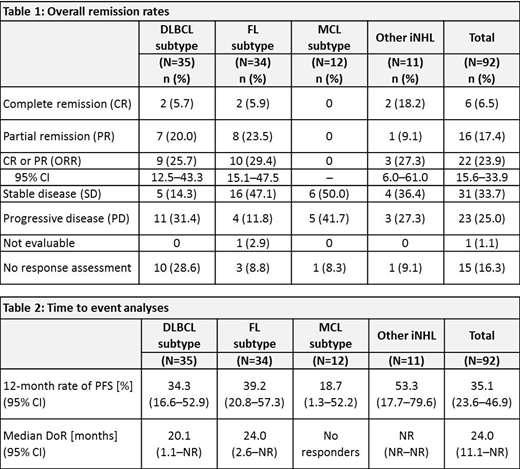
Results: The investigator-assessed best response (intent-to-treat analysis) in the different subgroups at cut-off date (28 Sep 2018) is shown in Table 1. Five patients in complete remission (CR) (one DLBCL, two FL, two other iNHL) were ongoing and still on tafasitamab treatment at the cut-off date. These patients were on treatment for more than 4 years. The median DoR was 20.1 months in DLBCL and 24 months in FL (Table 2). The median PFS was 2.7 (95% confidence interval [CI] 2.1-13.2 months) and 6.6 months (95% CI 5.3-20.5 months) in DLBCL and FL, respectively. The PFS rate at 12 months was 34.3% and 39.2% for DLBCL and FL, respectively (Table 2). Similar PFS was observed in rituximab-refractory as well as non-refractory patients. Patients with a peripheral blood natural killer (NK) cell count >100 cells/µL at baseline had a median PFS of 4.2 months (DLBCL) or 8.8 months (FL/iNHL), as compared with patients who had <100 NK cells/µL at baseline showing a median PFS of 2.1 months (DLBCL) or 3.2 months (FL/iNHL), respectively. Tafasitamab was well tolerated in patients with r/r NHL. Most treatment-emergent adverse events (TEAEs) were mild in nature. The most common grade ≥3 TEAEs were neutropenia (9.8%), thrombocytopenia (4.3%), anemia (3.3%) and pneumonia (3.3%). Four patients (4.3%) experienced serious adverse reactions (febrile neutropenia, genital herpes zoster, infusion-related reaction and myelodysplastic syndrome). There was no evidence of grade ≥3 late toxicity during the long-term follow-up period; no treatment-related deaths occurred.
Conclusion: Tafasitamab monotherapy until progression resulted in durable responses and was well tolerated in patients with both aggressive and indolent NHL subtypes.
Jurczak:Celgene: Research Funding; Bayer: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Research Funding; Servier: Research Funding; Sandoz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Research Funding; Roche: Research Funding; MorphoSys: Research Funding; Celtrion: Research Funding; Gilead: Research Funding; Loxo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; Incyte: Research Funding. Zinzani:Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Honoraria, Speakers Bureau. Hess:Janssen: Consultancy, Honoraria, Other: personal fees; Celgene: Consultancy, Employment, Honoraria, Other: personal fees, Research Funding; Roche: Consultancy, Employment, Honoraria, Other: personal fees, Research Funding; Pfizer: Other: personal fees, Research Funding; CTI: Consultancy, Employment, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria. Gaidano:AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra-Zeneca: Consultancy, Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sunesys: Consultancy, Honoraria. Provencio:Takeda: Consultancy, Other: Travel, accommodation and expenses, Speakers Bureau; Novartis: Consultancy, Other: Travel, accommodation and expenses, Speakers Bureau; AstraZeneca: Consultancy, Other: Travel, accommodation and expenses, Speakers Bureau; Pierre Fabre: Consultancy, Other: Travel, accommodation and expenses, Speakers Bureau; Boehringer Ingelheim: Consultancy, Other: Travel, accommodation and expenses, Research Funding, Speakers Bureau; Roche: Consultancy, Other: Travel, accommodation and expenses, Research Funding, Speakers Bureau; BMS: Consultancy, Other: Travel, accommodation and expenses, Speakers Bureau; MSD: Consultancy, Other: Travel, accommodation and expenses, Speakers Bureau. Nagy:Novartis: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Robak:Morphosys AG: Research Funding; BeiGene: Consultancy, Research Funding; Abbvie: Consultancy, Honoraria, Other: Travel grant, Research Funding; Gilead: Consultancy, Research Funding; Acerta: Research Funding; Janssen: Consultancy, Honoraria, Other: Travel grant, Research Funding; Amgen: Consultancy, Other: Travel grant; Roche: Consultancy, Other: Travel grant, Research Funding; Takeda: Consultancy, Research Funding; UCB: Honoraria, Research Funding. Maddocks:Teva: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Buske:Amgen: Research Funding; Bayer: Research Funding; Roche: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria; Celltrion: Honoraria, Speakers Bureau; Hexal: Honoraria, Speakers Bureau. Ambarkhane:MorphoSys: Employment. Brugger:MorphoSys: Employment; AstraZeneca: Equity Ownership. Dirnberger-Hertweck:MorphoSys: Employment. Tillmanns:MorphoSys AG: Employment. Weirather:MorphoSys: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal