Introduction: XmAb13676 is a humanized bispecific antibody that binds both CD20 and CD3 in order to recruit cytotoxic T cells to kill CD20 expressing malignant cells. Interim results of an ongoing first-in-human, dose-escalation study (XmAb13676-01; NCT02924402) in subjects with relapsed/refractory (R/R) non-Hodgkin's lymphoma (NHL) and chronic lymphocytic leukemia (CLL) are reported here.
Methods: The study is a first-in-human, multi-center, open-label, phase 1, dose-escalation study in subjects with R/R NHL and R/R CLL with a standard 3 + 3 design. The primary objectives are to determine safety, tolerability, and the maximum tolerated dose (MTD) or recommended dose of XmAb13676. Secondary objectives include preliminary anti-tumor activity and PK/PD of XmAb13676. This study is designed in two parts: Part A, escalating dose cohorts that establish an initial priming dose as part of repeated weekly infusions at a fixed dose in a 28-day cycle; and Part B, with a dosing schedule consisting of a priming dose on C1D1 , established in Part A, followed by escalated dose(s) on subsequent weeks. Cytokine Release Syndrome (CRS) prophylaxis with dexamethasone was mandated prior to each administration of XmAb13676. Treatment was continued for 2 cycles or longer if there was evidence of therapeutic benefit.
Results: At data cut-off, 44 subjects have been treated, 36 with NHL and 8 with CLL.
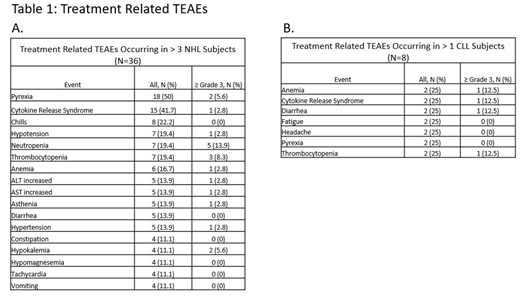
NHL: Subjects with R/R NHL had a median age of 61.5 years (range 32-89), a median of 3.5 prior therapies (range 1-9) and had been diagnosed a median of 24.6 months (range 6.3-181.2) prior to treatment in the study. Treatment-emergent adverse events (TEAEs) related to treatment occurring in ˃ 3 subjects are shown in Table 1A. Nine treatment-related serious adverse events (SAE) occurred in 6 subjects. The most common treatment-related SAE was CRS, which occurred in 4 (11.1%) subjects with 1 of the events being Grade 4 and the other events being ≤ Grade 2. Treatment responses were assessed by the Lugano criteria or International Working Group criteria for Waldenström Macroglobulinemia (WM). There have been 7 objective responses: 2 complete responses (CR; DLBCL), 1 Very Good PR (VGPR; WM), and 4 partial responses (PR; 1FL, 3 DLBCL) at doses of 20-125 µg/kg. In the efficacy-evaluable population, at doses of 80-125 µg/kg, objective responses were observed in 6/18 patients. A priming dose of 45 µg/kg has been chosen for Part B. An MTD has not been reached and dose escalation is ongoing in Part B in NHL.
CLL: Subjects with R/R CLL had a median age of 76 years (range 62-81), a median of 4.5 prior therapies (range 2-6) and had been diagnosed a median of 76.1 months (range 17.5-328.9) prior to treatment in the study. Treatment-related TEAEs occurring in ˃ 1 subject are shown in Table 1B. Three treatment-related serious adverse events (SAE) occurred in 2 subjects. The treatment related SAEs were CRS (Grade 3), hepatocellular injury (Grade 3), and jaundice cholestatic (Grade 2), each of which occurred in 1 (12.5%) subject. There has been 1 CR reported (Richter transformation) in 5 subjects at 20 µg/kg, the highest dose administered thus far. The treatment response was assessed by the Lugano criteria. An MTD has not been reached and dose escalation is ongoing in Part A in CLL.
Conclusions: XmAb13676 demonstrated evidence of clinical activity in heavily pretreated subjects with R/R NHL and R/R CLL treated at doses between 20 and 125 µg/kg. CRS was generally manageable with premedication. The study is ongoing with further optimization of dose and schedule.
Patel:AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Pharmacyclics/Janssen: Consultancy, Speakers Bureau; Sunesis: Consultancy. Chanan-Khan:AbbVie: Research Funding; Xencor: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Jansen: Research Funding; Mayo Clinic: Employment; Ascentage: Research Funding; Millennium: Research Funding. Salles:Novartis, Servier, AbbVie, Karyopharm, Kite, MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Epizyme: Consultancy, Honoraria; Roche, Janssen, Gilead, Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Autolus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Other: Educational events; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; BMS: Honoraria. Cartron:Gilead: Honoraria; Jansen: Honoraria; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Sanofi: Honoraria. Thomas:Celgene: Research Funding; Amgen: Research Funding; Xencor: Research Funding; BMS: Research Funding. Wierda:KITE pharma: Research Funding; Sunesis: Research Funding; Miragen: Research Funding; Gilead Sciences: Research Funding; Acerta Pharma Inc: Research Funding; GSK/Novartis: Research Funding; Cyclcel: Research Funding; Loxo Oncology Inc.: Research Funding; AbbVie: Research Funding; Genentech: Research Funding; Juno Therapeutics: Research Funding; Oncternal Therapeutics Inc.: Research Funding; Pharmacyclics LLC: Research Funding; Xencor: Research Funding; Janssen: Research Funding. Liebowitz:Xencor: Employment, Equity Ownership. Pagel:AstraZeneca: Consultancy; Gilead Sciences: Consultancy; Pharmacyclics: Consultancy. Ribrag:MSD: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel, accommodations, and expenses ; Nanostring: Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Research Funding; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; AZ: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses ; Incyte: Membership on an entity's Board of Directors or advisory committees; ArgenX: Research Funding. Saville:Xencor: Employment, Equity Ownership. Johnson:Xencor: Employment, Equity Ownership. Ly:Xencor: Employment, Equity Ownership. Phillips:Pharmacyclics: Consultancy, Research Funding; Bayer: Consultancy; Abbvie: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Seattle Genetics: Consultancy; Gilead: Consultancy; Incyte: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal