Background: Angioimmunoblastic T-cell lymphoma (AITL) is a relatively common subtype of peripheral T-cell lymphoma (PTCL) that typically presents with lymphadenopathy, extranodal disease, including rash, and is associated with frequent infections due to immune dysregulation. Patients with AITL generally have a poor prognosis, even with aggressive chemotherapy as responses to standard chemotherapy are often suboptimal. Recent advances in cancer biology suggest that AITL is derived from T-follicular helper cells and is often characterized by gross epigenetic dysregulation. Histone deacetylase (HDAC) inhibitors have demonstrated significant activity in T-cell neoplasms. The BELIEF trial established an overall response rate of 25% in patients with relapsed/refractory PTCL who were treated with belinostat, with a duration of response of about 1 year, leading to accelerated approval. Herein, we present a subset analysis of the data for patients with AITL.
Methods: Patients with histologically confirmed PTCL (N = 129) who experienced failure with or refractory to ≥ 1 prior systemic therapy received belinostat 1,000 mg/m(2) as daily 30-minute infusions on days 1 to 5 every 21 days. Central assessment of response used International Working Group criteria. Primary endpoint was overall response rate (ORR). Secondary endpoints included duration of response (DoR) and progression-free and overall survival (PFS).
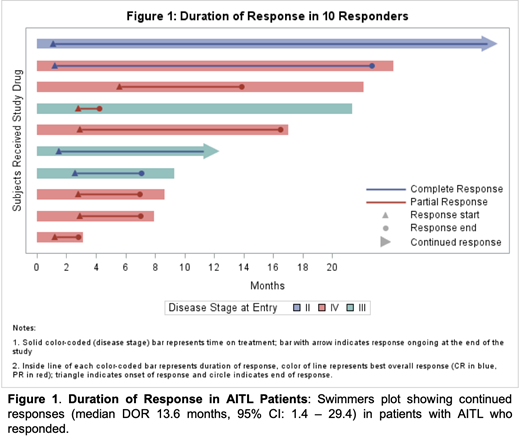
Results: Of 129 patients, 22 patients had AITL; most had advanced disease (91% stage III/IV; 36% with bone marrow involvement). The median number of prior therapies was 2 (range, 1-5), and 3 (14%) patients were refractory to their last line of therapy. The ORR for patients with AITL was 46% (10/22; 95%CI: 24 - 68%), with a complete response (CR) in 4 of 22 patients (18%). Of the ten responders, the median time to response of 11.3 weeks (range, 4.7 - 24.4 weeks) in the AITL subgroup. After a median follow up of 21.5 months, the median PFS was 4.2 months (95%CI: 1.5 -13.9) and the median DOR was 13.6 months (95%CI: 1.4 - 29.4) as shown in Figure 1. For all patients with AITL treated with belinostat, the median OS was 9.2 months (95%CI: 6.8 - 21.5). The most common grade 3 to 4 adverse events were asthenia (n=2), fatigue (n=2), anemia (n=2), thrombocytopenia (n=2), neutropenia (n=2), and septic shock (n=2).
Conclusions: Single-agent belinostat induced rapid and durable responses in patients with relapsed/refractory AITL. At the end of the study, there were 37% patients with ongoing responses at 2 years. Patients with clinical benefit from belinostat continued treatment until progression of disease. These results support the use of belinostat in relapsed/refractory AITL as a single agent and provide rationale for combination therapies in clinical trials.
Sawas:Seattle Genetics, Gilead, Daiichi Sanko: Consultancy; Affimed: Research Funding. Shustov:Spectrum Pharmaceuticals: Consultancy, Research Funding. Hsu:Spectrum Pharmaceuticals: Employment. Bhat:Spectrum Pharmaceuticals: Employment. Acosta:Acrotech Biopharma: Employment. Horwitz:Astex: Consultancy; Kyowa Hakko Kirin: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Innate Pharma: Consultancy; Kyowa Hakko Kirin: Consultancy; Trillium: Research Funding; Affimed: Consultancy; ADCT Therapeutics: Research Funding; Kura: Consultancy; ADCT Therapeutics: Research Funding; Aileron: Research Funding; Seattle Genetics: Consultancy, Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trillium: Research Funding; Aileron: Research Funding; Trillium: Research Funding; Miragen: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Kura: Consultancy; Forty-Seven: Research Funding; Millennium/Takeda: Consultancy, Research Funding; ADCT Therapeutics: Research Funding; Mundipharma: Consultancy; Kura: Consultancy; Miragen: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Consultancy; Astex: Consultancy; Seattle Genetics: Consultancy, Research Funding; Astex: Consultancy; Portola: Consultancy; Celgene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Aileron: Research Funding; Trillium: Research Funding; Forty-Seven: Research Funding; Infinity/Verastem: Consultancy, Research Funding; Innate Pharma: Consultancy; Miragen: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Mundipharma: Consultancy; Portola: Consultancy; Mundipharma: Consultancy; Portola: Consultancy; Aileron: Research Funding; Forty-Seven: Research Funding; Kura: Consultancy; Kyowa Hakko Kirin: Consultancy; Seattle Genetics: Consultancy, Research Funding; Portola: Consultancy; ADCT Therapeutics: Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Consultancy; Innate Pharma: Consultancy; Kyowa Hakko Kirin: Consultancy; Miragen: Consultancy; Affimed: Consultancy; Affimed: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Innate Pharma: Consultancy; Seattle Genetics: Consultancy, Research Funding; Forty-Seven: Research Funding; Affimed: Consultancy; Millennium/Takeda: Consultancy, Research Funding. O'Connor:Mundipharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADCT Therapeutics, Affimed, Agensys, Merck, Seattle Genetics, Spectrum, Trillium, and Verastem Oncology.: Research Funding; TG Therapeutics: Other: Travel Support, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal