Background
Effective treatment for relapsed, refractory (R/R) mantle cell lymphoma (MCL) post Bruton's Tyrosine Kinase inhibitor (BTKi) therapy represents an unmet clinical need with studies consistently reporting dismal outcome. No treatment strategy demonstrates superiority and there is currently no consensus on management.
R-BAC (Rituximab, Bendamustine and Cytarabine) has demonstrated excellent upfront response rates in phase 2 trials (overall response rate (ORR) 100%, 2-year progression free survival (PFS) 95%, Visco et al 2013), but with durable responses also reported with other combination therapies there is a rationale to reserve R-BAC for post-BTKi relapse.
Aims
There is currently no data available assessing efficacy of R-BAC in the post BTKi setting. We first adopted this treatment strategy within routine clinical practice in 2015 and in this study have collected and analysed clinical outcomes across 20 centers in the United Kingdom and Italy.
Methods
35 R/R MCL patients (pts) with prior BTKi therapy started R-BAC between October 2015 and February 2019. Treatment consisted of rituximab (375 mg/m2 or 500 mg) D1, bendamustine 70 mg/m2 D1 and D2 and cytarabine 500 mg/m2 D1 to D3 in a 28 day cycle. 29 pts were treated in the UK and 6 in Italy. Analysis included one pt with previous alloHSCT. Response to therapy was measured using Lugano classification (Cheson et al, 2014), although assessment of CR with bone marrow biopsy was not always undertaken. Baseline data, including response to previous BTKi, was collected retrospectively by the treating physician. The primary outcome was PFS to R-BAC.
Results
Median age at time of R-BAC was 66.3 years (range 43 to 81) and 82.9% of pts were male. At initial diagnosis MIPI was 34.5% low risk, 17.2% intermediate risk and 48.3% high risk; histology was 20.6% blastoid. Pts received a median 2 prior lines of systemic therapy (range 1 to 6). Frontline therapy included high dose cytarabine containing regimen (61.7%) plus consolidation AutoSCT (40.0%), R-CHOP (26.4%), R-CVP (2.9%), FC (2.9%) and ibrutinib plus rituximab (5.9%). 7 patients received maintenance rituximab (28.6%). 45.7% of patients commenced second line therapy within 2 years of initial diagnosis. Prior BTKi therapy included: ibrutinib (n=30), acalabrutinib (n=2), tirabrutinib (n=2) and M7583 (n=1). ORR to prior BTKi was 67.6% (CR 35.3%) and median PFS was 9.2 months. All patients stopped BTKi therapy due to progressive disease (94.3%) or failure to respond (5.7%).
All but 1 patient received R-BAC directly after relapse on BTKi. Patients received a median of 4 cycles of treatment (range 1 to 6). 9 pts received attenuated doses of chemotherapy at clinician's discretion from start of therapy and 13 additional pts received attenuated doses beyond cycle 1 (62.9% of all patients received some form of dose attenuation).
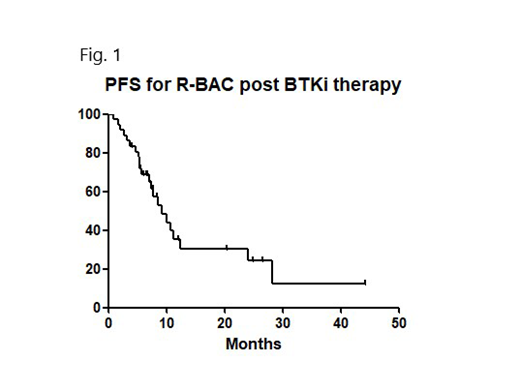
ORR to R-BAC was 82.3% (CR/CRu rate 55.9%), median PFS 9.3 months (see fig. 1) and median OS 12.2 months. Importantly, outcome for 11 pts ≥70 yrs was similar to younger pts (median PFS 10.6 months vs 8.6 months, p=0.83). 53.5% of evaluable patients demonstrated longer duration of response compared with preceding BTKi.
25 pts completed the planned treatment course, 2 stopped early due to excess toxicity (prolonged cytopenias and infection) and 8 stopped early due to progressive disease. 9 pts received subsequent consolidation alloSCT, and 1 DLI. Although follow-up is short only 1 patient to receive alloSCT has relapsed. 13 patients overall remain in remission, including 5 beyond 12 months.
18 patients (51.4%) required admission during R-BAC, including 15 with neutropenic fever (42.8%) and 72.7% patients required transfusion support. There were no treatment related mortalities.
Conclusion
This high risk population with a short PFS to prior BTKi demonstrated an excellent response rate to R-BAC. Favorable outcomes in the cohort consolidated with alloSCT, and the generally short duration of response in other pts, suggests R-BAC can be primarily used as a bridge to alloSCT in suitable pts. Treatment had notable hematological toxicity but with efficacy maintained in older pts R-BAC remains a valid option in those deemed unsuitable for transplant, although judicious dose attenuation is advised.
In an area lacking a clear therapeutic path, the results from our study support R-BAC being considered a new standard of care option for R/R MCL in the post BTK inhibitor setting.
Frewin:AbbVie: Other: Meeting attendance sponsorship ; Novartis: Consultancy, Other: Meeting attendance sponsorship . Eyre:Roche: Honoraria; Abbvie: Honoraria; Gilead: Consultancy, Honoraria, Other: commercial research support; Janssen: Honoraria. Lambert:Takeda Pharmaceuticals: Other: Funding to attend a scientific conference in 2018. Crosbie:Janssen: Honoraria. Rule:Janssen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Astra-Zeneca: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Kite: Consultancy; Pharmacyclics: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Sunesis: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Napp: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal