In acute myeloid leukemia (AML), blasts lose their ability to differentiate into mature cells and to undergo apoptosis. Accordingly, a proapoptotic and differentiating therapy (arsenic and retinoic acid) has dramatically improved survival in acute promyelocytic leukemia; however, a similar combination therapy is not available for other AML subtypes. In 2016, inhibition of dihydroorotate dehydrogenase (DHODH), a key enzyme of the pyrimidine biosynthesis, was found to induce differentiation in several AML models; for in vivo studies, brequinar (BRQ) was utilized. Starting from BRQ and applying a scaffold-hopping replacement, we have recently developed a new DHODH inhibitor, Meds433, which could induce differentiation at a 1-log lower concentration compared to BRQ (Sainas, J Med Chem 2018). Here we characterize Meds433 with in vitro and in vivo experiments, showing that it has a significant pro-apoptotic effect in several AML cell lines, which is at least partially independent from the differentiating effect.
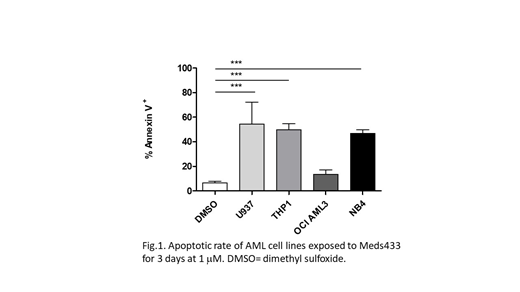
Analyzing the kinetic of differentiation induced by Meds433 on U937 and THP1 cell lines, and comparing the data with the number of viable cells, we noticed that cells started to die before the differentiation effect could be significant. Hence, we decided to investigate the proapoptotic effect of Meds433, treating several AML (U937, THP1, OCI-AML3, NB4) and non-AML (CEM, P3j, peripheral blood mononuclear cells-PBMC) cell lines with Meds433, and analyzing the expression of Annexin V and propidium in flow cytometry. Experiments demonstrated that Meds433 had a significant pro-apoptotic effect on several AML cell lines (Fig.1), but not on non-AML cell lines. The apoptotic rate increased with the time of exposure (3 vs 6 days), allowing to obtain a good apoptotic rate also in OCI-AML3, the most resistant cell line in our hands. As for the differentiation experiments, Meds433 could induce apoptosis at a 1-log inferior concentration compared to BRQ. More interestingly, in NB4 cells, a strong apoptotic effect was not associated with any differentiating feature, indicating that DHODH inhibition can induce apoptosis directly. As NB4 is a promyelocytic cell line, we also compared the effects of ATRA (all-trans retinoic acid), Meds433 and their combination. ATRA alone was found to induce strong differentiation and mild apoptosis on NB4 cells, while Meds433 alone could induce exclusively apoptosis; finally, the combination of ATRA and Meds433 increased both the rate of differentiating and apoptotic cells compared to ATRA only.
We next tried to further characterize the apoptotic effect. When experiments were performed in the presence of uridine, a downstream product of DHODH in the pyrimidine biosynthesis, the apoptotic effect was totally abrogated. This phenomenon was already observed in the differentiation experiments (Sainas, J Med Chem 2018), and it confirms that both differentiation and apoptosis are indeed caused by the pyrimidine depletion rather than off-target mechanisms. Moreover, when experiments were performed in hypoxic conditions, the rate of apoptosis did not change, suggesting that Meds433 could work in the bone marrow hypoxic niche of (leukemic) stem cells. Further analyzing the effect of Meds433 on non-AML cells, we evaluated the maturation of T-lymphocytes, from naïve to TEMRA (T effector memory RA), finding no influence at all.
Finally, preliminary results from in vivo experiments show that i) Meds433 is not toxic on Balb/c mice after 5 weeks of intraperitoneal administration; ii) the half-life is limited to 4-6 hours and iii) Meds433 has a good antileukemic activity (approximately 50% reduction of the tumor volume compared with control, after an 18-day treatment of THP1-xenograft in NSG mice).
In conclusion, our work demonstrates that: i) DHODH inhibition can induce apoptosis in AML cells both directly and as a result of differentiation, partially depending on the cell line; ii) Meds433 is a novel, promising antileukemic drug, with limited toxicity, which could be active on a wide variety of AML. Since continuous exposure to the drug is fundamental in the pyrimidine starvation strategy, we are currently optimizing Meds433 pharmacokinetic profile in order to maximize the in vivo antileukemic activity.
Saglio:BMS: Consultancy; Novartis: Consultancy; Ariad: Consultancy; Incyte: Consultancy; Pfizer: Consultancy; Jansen: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal