Introduction: C-type lectin 1 (CLL-1, CD371) is highly expressed on the malignant cells from many patients with AML, and CAR T cells directed to this antigen can selectively target both leukemic progenitor cells (LSC) as well as AML blasts whilst sparing normal tissues. We previously showed (1) that such CAR-Ts can recognize and eliminate both AML blasts and primitive AML colony-forming cells in a low tumor-burden model. We have now modified the structure of the CLL-1 CAR and added transgenic expression of IL15 to enhance performance sufficiently for activity even against more extensive disease.
Material and Methods: We assessed the phenotype and cytolytic ability of T cells transduced with 5 CLL-1 CAR constructs, varying in their spacer, transmembrane and costimulatory sequences (CD28z-CD8, CD28z-sh, CD28z-CH3, 4-1BBz-sh, 4-1BBz-CH3), and compared these with the effects of our published construct (4-1BBz-CD8)(1). We used flow cytometry to determine the effects of each construct on T cell phenotype and differentiation, and sequential (recursive) co-culture assays with tumor-cell targets to determine the durability of the anti-tumor activity. The most active constructs (CD28z-CD8 and 4-1BBz-CD8) were then evaluated in NOD.SCID IL-2Rg-/- (NSGS) mice engrafted with 1.5x10ˆ6 FFLuc-modified HL 60 AML cells, which received 2x10ˆ6 CLL-1 CAR T cells on day 3. To determine if we could further potentiate the in vivo expansion, persistence and anti-tumor activity of the CLL-1 CAR-T cells, we used a second retroviral vector to co-express transgenic IL15, measuring the effects in vitro and in vivo. Mice engrafted with 1.5x10ˆ6 tumor cells and received 2.5x10ˆ6 CLL-1 CAR T cells on week 3 in patient derived xenograft (PDX) model. We determined antitumor activity by bioluminescence imaging and weekly bleeding and measured serum cytokines by multiplex analysis (Luminex, TX). After euthanasia, we examined formalin-fixed/paraffin embedded sections.
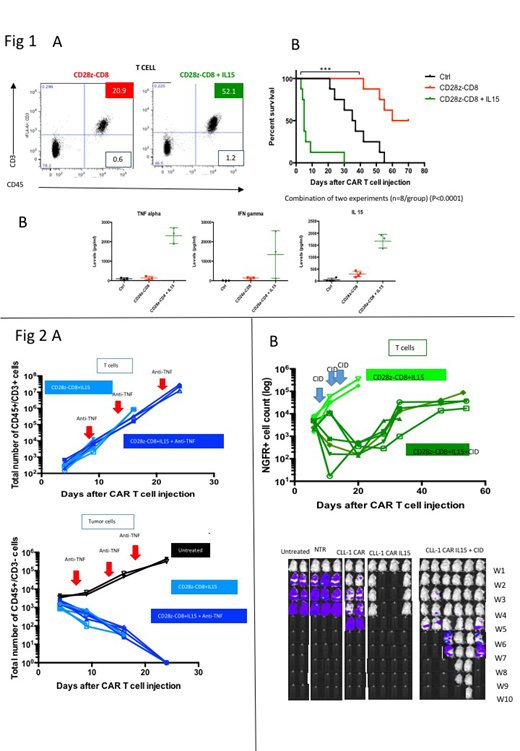
Results: Modified CLL-1 CAR constructs were expressed by 70-80% of cells irrespective of CAR sequence, but CD28z-CD8 CAR T cell expansion was significantly higher than CAR T cells with 4-1BBz endodomains (p<0.001), in part because of a higher death rate/lower viability in 4-1BBz cells (p<0.001). Consistent with these differences, both CD4 and CD8 T cell populations had more terminally differentiated cells (CCR7-CD45RA+) in CD28z versus 41BBz CAR T cells. In sequential co-culture assays against HL 60 (E:T=1:4) and THP-1 (E:T=1:4), CD28z-CD8 CAR T cells continued to expand well producing the greatest antitumor effect. In vivo models showed reduction in tumor signal in mice receiving either CD28z-CD8 CAR T or 4-1BBz-CD8 CAR T cells, but that only CD28z-CD8 CAR T cells prolonged survival (p<0.01). Nonetheless, all mice ultimately relapsed, usually with extramedullary disease, in association with limited CAR T persistence. We therefore incorporated transgenic IL15 as a "signal 3" for CD28z-CD8 CAR T cells, and determined the effects of forced IL15 expression on T cell phenotype, expansion, and antitumor activity in vitro and in vivo. In vitro, CD28z-CD8 CAR T cells with IL15 were less terminally differentiated and had superior expansion compared to CD28z-CD8 CAR T cells without IL15 (p<0.001). In both AML PDX and AML cell line animal models, CD28z-CD8 CAR T co-expressing transgenic IL15 initially (week 1) expanded better than CD28z-CD8 CAR T without IL15 (p<0.001) (Fig 1a), but produced severe acute toxicity associated with high level production of human IL15, TNF alpha and IFN gamma (Fig 1b). Histopathology showed marked inflammatory changes with tissue damage in lung and liver. This acute toxicity could be managed by 2 strategies, individually or in combination. The excessive TNF alpha secretion could be blocked with anti-TNF alpha antibody (1mg/kg/mouse) (BioLegend, CA USA) weekly, while excessive T cell expansion could be arrested by activation of an inducible caspase 9 safety switch by administration of dimerizing drug (2). Both strategies successfully prolonged tumor free survival (Fig 2,b).
Conclusion: Addition of transgenic IL15 to CLL-1-CD28z-CD8 CAR augmented activity against AML in a range of cell line and PDX models, and toxicity associated with exuberant CART expansion could be prevented by cytokine blockade and/or an inducible safety switch.
References:
1. Tashiro H, et al. Mol Ther. 2017
2.Straathof KC et al. Blood. 2005
Brenner:T Scan: Membership on an entity's Board of Directors or advisory committees; Marker Therapeutics: Equity Ownership; Allovir: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Equity Ownership; Memgen: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal