Background: The PD-1/PD-L1 axis is a dominant cancer immune escape pathway, and PD-1 blockade therapy has greatly benefited patients with select solid tumors and lymphomas. Unfortunately, anti-PD-1 monotherapy has limited efficacy against relapsed/refractory (r/r) diffuse large B cell lymphoma (DLBCL) - a disease where new therapies are needed. Because numerous inhibitory checkpoint receptors have been implicated in driving tumor-specific T cell dysfunction, we hypothesized that combinatorial checkpoint blockade therapy (CBT) would enhance the activity of PD-1-based therapy in r/r DLBCL. T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) is a recently identified co-inhibitory receptor expressed on dysfunctional tumor-infiltrating T cells. PD-1 and TIGIT co-blockade therapy has demonstrated impressive activity in pre-clinical solid tumor and myeloma models. However, the degree to which TIGIT is involved in mediating T cell suppression in DLBCL is not fully known.
Methods: TIGIT expression on lymphoma-infiltrating T cells (LITs) from 18 fresh lymphoma samples was analyzed by flow cytometry. Multiplex IHC on tissue microarrays (TMAs) was also performed to investigate TIGIT expression in DLBCL samples. The syngeneic murine A20 B cell lymphoma model was employed to study: 1) the kinetics of TIGIT and other co-receptor expression on LITs, 2) the association of TIGIT expression with effector function among LITs, and 3) the effectiveness of anti-TIGIT mono- and combination CBT in mice with established A20 lymphomas. A20 lymphoma tumors were established in groups of Balb/c mice by subcutaneous (SQ) injections of 5 x 106 cells. Expression of TIGIT and other co-receptors in A20 LITs was examined by flow cytometry at various time points. Function of TIGIT+ LITs was assessed by examining cytokine production following ex vivo stimulation with PMA and ionomycin. To test the efficacy of TIGIT blockade, mice received intraperitoneal injections of anti-TIGIT, anti-PD-1, anti-4-1BB, or combinations of these antibodies. Treatments began once tumors reached a diameter of 10 mm and were continued every 3 days for 5 doses. Tumor growth was monitored and compared to that in A20-bearing mice treated with isotype control antibodies. In some experiments, mice that achieved complete tumor rejection following single or dual CBT were re-challenged with A20 cells to investigate immunological memory responses.
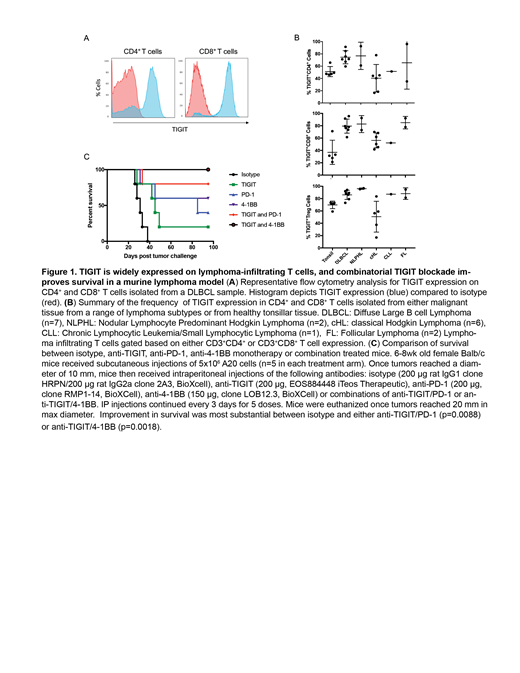
Results: Across a variety of human lymphomas, flow cytometric analysis revealed that TIGIT was broadly upregulated on LITs, including regulatory T cells and conventional CD4+and CD8+ T cells (Figure A and B). TIGIT expression on LITs in DLBCL was particularly high. Nearly all TIGIT+ LITs were also PD-1+, suggesting that these receptors co-orchestrate a T cell dysfunctional state in the lymphoma environment. Multiplex immunofluorescence staining of DLBCL samples demonstrated that TIGIT was most highly expressed on CD8+ T cells and that TIGIT+ T cells tended to be localized near, and in some cases, surrounding CD20+ lymphoma cells. Consistent with observations in human lymphomas, LITs isolated from murine A20 lymphoma commonly co-expressed TIGIT and PD-1, and the degree of expression correlated directly with tumor volume. This correlation was also present for other co-receptors, including 4-1BB, TIM3, and CTLA-4. Ex vivo restimulation of A20 LITs revealed that TIGIT+ T cells produced lower levels of effector cytokines, such as TNF-α, compared with TIGIT- T cells. In mice with established A20 lymphomas, both TIGIT and PD-1 mono-blockade led to modest delays in tumor outgrowth compared with mice treated with isotype control antibodies. Strikingly, however, combined PD-1 and TIGIT blockade resulted in complete rejection of A20 lymphomas in most mice and led to significantly prolonged survival compared to mice treated with single agent CBT (Figure C). Combination TIGIT and 4-1BB CBT was also remarkably effective in driving rejection of A20 lymphomas, and led to remarkable memory responses.
Conclusions: TIGIT promotes immune tolerance in the DLBCL environment. While TIGIT monotherapy has anti-lymphoma activity, combinatorial CBT incorporating anti-TIGIT antibodies drives extremely potent rejection of established lymphomas in mice. These results provide rationale for further study of TIGIT blockade as a therapeutic strategy in r/r lymphomas, including DLBCL.
Sunseri:iTeos Therapeutics: Research Funding. Wald:iTeos Therapeutics: Employment. Preillon:iTeos Therapeutics: Employment. Smith:Portola Pharmaceuticals: Research Funding. Driessens:iTeos Therapeutics: Employment. Kline:Merck: Honoraria; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal