
Background: Although daunorubicin/cytarabine ("3+7")-based chemotherapy (CT) regimens produce complete remission (CR) rates of approximately 70% in younger adults, about 30% require re-induction. Among those achieving CR, relapse after post-remission intensive CT or allogeneic transplant is common and limits cure. Venetoclax (VEN) is an orally available selective BCL-2 inhibitor that promotes mitochondrial-mediated apoptosis in myeloblasts during cytotoxic stress. VEN in combination with hypomethylating agents and low dose cytarabine in untreated unfit elderly AML patients (pts) elicits high response and led to drug approval in that setting. VEN in combination with standard '3+7" could lead to a higher rate of measurable residual disease (MRD)-negative CR, perhaps obviating the need for reinduction as well as limiting relapse after consolidation therapy without undue toxicity.
Methods: The primary objective of this phase 1 study is to determine the maximum tolerated dose of VEN that can be given in combination with standard intensive induction (ind) and consolidation CT in previously untreated AML pts. Pt eligibility includes no prior treatment (rx) for AML, FLT3 wild type, non-inv16 or t(8; 21) AML, either de novo or secondary. Pts must not require strong CYP3A inhibitors/inducers, have normal organ function, have WBC<25 K at treatment start (hydroxyurea allowed before protocol rx), and be age >=18 (study amended to include only those age 18-60 after the first 6 pts were treated). VEN is given during ind on days 1-11 in cohorts of 200, 400, and 600 mg/d/ (after 4 d ramp-up) with daunorubicin 60 mg/m2/d IVP days 2-4 and cytarabine 200 mg/m2/d by IVCI days 2-8. A day 15 marrow determines the need for a second ind consisting of the same doses of VEN, daunorubicin and cytarabine given on days 1-8, 2-3, and 2-6, respectively. VEN escalation is carried out in 3+3 fashion. Once the MTD for VEN during ind is determined pts will be treated at that dose during ind and remitting pts given escalating doses (cohorts of 200, 400, 600 mg) of VEN d1-8 in a 3+3 fashion (beginning at one dose level below the MTD (or 100 mg if 200 mg is determined to the ind MTD)) with a single cycle of cytarabine 3g/m2 over 3h q 12 h on days 1, 3, and 5. A 10 pt confirmation cohort will be accrued at the MTD. Dose-limiting toxicity (DLT) is defined as any death, delayed neutrophil recovery (failure to achieve absolute neutrophil count (ANC) ≥ 500/mL by day 42 in pts without residual AML), or any gr 4 non-heme non-resolving toxicity not clearly due to CT alone. A marrow exam, including an assessment of MRD by multiparameter flow cytometry with a sensitivity of 0.02%, is carried out at the time of count recovery during induction but no later than day 42. Correlative studies include flow cytometry-based BH3 profiling (Vo TT, Cell, 2012) on blasts obtained at enrollment and just prior to chemo and CyTof to characterize BCL-2-associated proteins.
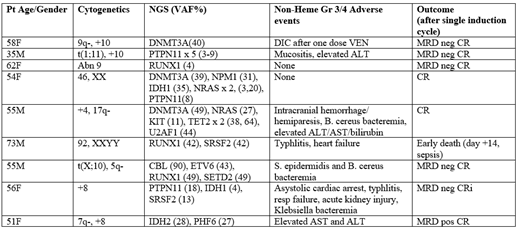
Results: The first pt treated (58 yo F with normal karyotype, and NGS panel showing a DNMT3A mutation, though subsequent PCR-based assay disclosed a FLT3 ITD) developed gr 4 DIC after the first dose of VEN 50 mg (required factor replacement for elevated INR, but had no bleeding). VEN was stopped and she achieved an MRD negative CR with standard ind, but the first cohort was expanded to 6 pts. 4 of the 5 additional pts achieved a CR, but a 73 year old man died of nadir sepsis at d 14. Given 2/6 DLT events, VEN 200 was considered unacceptable by protocol design. An amendment allowed further accrual at the starting dose of VEN 200 mg but restricted accrual to age 18-60. All 3 pts on the expanded cohort have responded, and none experienced a DLT, so accrual to the VEN 400 mg cohort is now beginning. None of the pts required a second ind course and no tumor lysis was seen. The median time to ANC and platelet recovery to 1K/ul and 100K/ul in the 7 pts who received all planned VEN is 36 d (range 24-43) and 27 d (range 24-55), respectively. The table lists the specific pt characteristics, adverse events and outcome.
Conclusions: We have preliminarily shown that VEN 200 mg/d beginning one d prior and until three d after 3+7 ind can be given safely in pts age 18-60; escalation to VEN 400 mg is ongoing. Flow based MRD-negative remissions have been noted after a single ind cycle. Plans are underway to compare the VEN/3+7 ind regimen derived from this trial to 3+7 in non-favorable risk newly diagnosed AML pts ages 18-60 in the context of a North American Intergroup study.
Stone:Novartis: Consultancy, Research Funding; Macrogenics: Consultancy; Astra-Zeneca: Consultancy; Arog: Consultancy, Research Funding; Arog: Consultancy, Research Funding; Otsuka: Consultancy; Agios: Consultancy, Research Funding; Actinium: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Other: DSMB; Daiichi-Sankyo: Consultancy; Astellas: Consultancy; Otsuka: Consultancy; Biolinerx: Consultancy; Argenix: Other: DSMB; Amgen: Membership on an entity's Board of Directors or advisory committees; Argenix: Other: DSMB; Stemline: Consultancy; Biosight: Consultancy; Roche: Consultancy; Takeda: Other: DSMB; Novartis: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Pfizer: Consultancy; Trovagene: Consultancy; Biolinerx: Consultancy; Jazz: Consultancy; Trovagene: Consultancy. DeAngelo:Incyte: Consultancy; Amgen: Consultancy; Jazz Pharmaceuticals Inc: Consultancy; Celgene: Consultancy; GlycoMimetics: Research Funding; Blueprint: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Shire: Consultancy; Takeda Pharmaceuticals: Consultancy; Abbvie: Research Funding. Galinsky:Merus Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; AbbVie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; ABIM: Other: Member on specialty oncology board. Letai:AbbVie, AstraZeneca, Novartis: Consultancy, Research Funding; Zeno Pharmaceuticals, Vivid Bioscience, Flash Therapeutics, Dialectic Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Cofounder or Advisory Board member. Konopleva:Kisoji: Consultancy, Honoraria; Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding.
Venetoclax is being used off label in combination with standard and induction and consolidation chemotherapy in AML
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal