BACKGROUND: Despite recent advances, outcomes remain poor in secondary and relapsed/refractory (R/R) acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). We previously demonstrated that combination thioguanine (6TG) and decitabine restores therapeutic efficacy in vitro in R/R AML (O'Dwyer K et al. Blood 2010;114:2657). Based on these data, we completed a Phase I dose-escalation study of 6TG with decitabine in advanced myeloid malignancies with an overall response rate (ORR) of 67% and median progression-free survival (PFS) of 42 weeks in responding patients (Lee DJ, et al. Blood 2016;128:2816). Moreover, 5 of the 6 study participants who had previously received a hypomethylating agent (HMA) responded, suggesting that 6TG in combination with decitabine can overcome HMA resistance. Because of these results, a cohort of patients with high-risk and R/R AML and MDS were treated with combination 6TG and decitabine at Columbia University Irving Medical Center, and we now report our clinical experience with this regimen.
METHODS: A retrospective chart review was performed of all adult patients at our institution with advanced myeloid malignancies who had received at least one cycle of 6TG and decitabine between 2013-2017. The treatment regimen utilized the maximum tolerated dose of 6TG identified in the phase I trial. Up to 2 cycles of induction with 6TG 80 mg/m2/day PO in 2 divided doses was given on Days 1-12. Decitabine 20 mg/m2 IV was given on Days 3-12. Following this, maintenance cycles consisted of 6TG on Days 1-7 and decitabine on Days 3-7 at the same doses. Treatment was continued until the time of hematopoietic stem cell transplant (HSCT), disease progression, or toxicity. The primary objective of this retrospective analysis was to determine the ORR. Secondary objectives were to evaluate PFS and overall survival (OS) in this population.
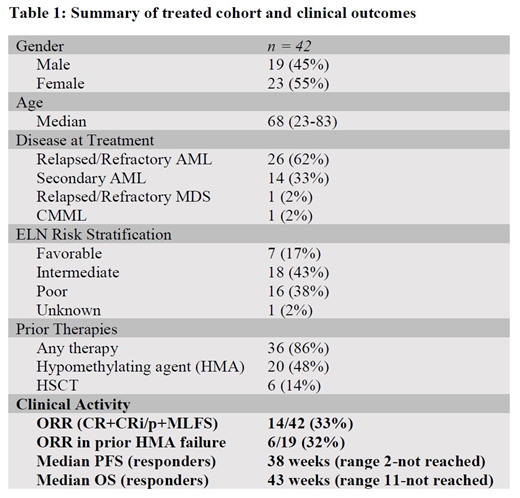
RESULTS: Forty-two patients were identified, 55% of whom were female, with a median age of 68 years (range, 23-83 years). Thirty-three percent of the patients had secondary AML; 62% had R/R AML; 2% had R/R MDS; and 2% had chronic myelomonocytic leukemia. By ELN genetic risk stratification, 38% were adverse-risk, 43% were intermediate, and 17% were favorable, while 2% were unknown. However, 36 (86%) patients failed prior therapy, suggesting that nearly all of the treated patients were poor-risk. Nineteen (45%) patients had received prior HMA therapy, and 6 (14%) had undergone previous HSCT. Patients received a median of 2 cycles of therapy (range, 1-10). Five (12%) patients achieved complete remission (CR), 7 (16%) obtained a CR with incomplete count recovery (CRi/p), and 2 (5%) had morphologic leukemia-free state (MLFS), for an ORR of 33% (CR+CRi/p+MLFS). One notable patient who achieved CR was a 73-year-old with secondary AML, complex cytogenetics, and a TP53 mutation who subsequently went on to HSCT. Another 5 (36%) responders had complex karyotypes or poor-risk genetics. Ten of the 14 responders (71%) failed prior therapy. Of the 19 who had received prior HMA therapy, 6 (32%) responded. Six of 14 (43%) responders proceeded to HSCT. Two additional patients had significant blast reduction and underwent HSCT. Responses and maximum blast reduction were obtained after two cycles of therapy. For the responders, the median PFS was 38 weeks (range, 11-not reached), while the median OS was 43 weeks (range, 11-not reached).
CONCLUSIONS: Combined 6TG and decitabine is a highly active regimen in advanced myeloid malignancies, with an ORR of 33% in this cohort, and median PFS and OS of 38 and 43 weeks, respectively, in responding patients. This compares favorably to the expected response rate of 10-20% with decitabine monotherapy in this poor-risk population consisting of mostly secondary and R/R AML. These data also confirm our prior phase I study findings that the addition of 6TG to decitabine can overcome prior HMA resistance, with 32% of those who had previously failed HMA therapy responding. This combination remains a viable therapeutic option for elderly and unfit patients with high-risk and R/R AML who cannot tolerate intensive chemotherapy.
Heaney:BMS: Research Funding; Partner Therapeutics: Consultancy; Roche: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Celgene: Research Funding; Novartis: Consultancy; AbbVie: Consultancy; Deciphera: Research Funding; Constellation: Research Funding; Blueprint: Research Funding; CTI: Research Funding. Lamanna:Ming: Research Funding; Celgene: Consultancy; Oncternal: Research Funding; TG Therapeutics: Research Funding; Infinity/ Verastem: Research Funding. Jurcic:Syros Pharmaceuticals: Research Funding; Astellas: Research Funding; AbbVie Inc: Consultancy; Daiichi Sankyo: Research Funding; Celgene: Consultancy, Research Funding; Incyte: Consultancy; Kura Oncology: Research Funding; Forma Therapeutics: Research Funding; Genentech: Research Funding; Novartis: Consultancy; Actinium Pharmaceuticals: Research Funding. Frattini:Celgene: Employment, Equity Ownership; Lin Bioscience: Consultancy. Lee:Abbvie: Research Funding; Roche: Research Funding; Tolero: Research Funding; Forty Seven, Inc.: Research Funding; Bayer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal