BACKGROUND: Venetoclax is a potent, oral BCL-2 inhibitor that results in rapid clearance of acute myeloid leukemia (AML) blasts leading to cytoreductions after initiation of treatment. Venetoclax in combination with hypomethylating agents (HMAs) or low dose cytarabine (LDAC) results in a high rate of response in patients with AML. For this reason, management of neutropenia after achievement of marrow response during venetoclax-based treatment, including dose delays, dose reductions, and supportive care are collectively important for optimizing patient outcomes. Here, management of neutropenia is analyzed during treatment with venetoclax in combination with HMA or LDAC.
METHODS: This analysis includes patients from the open-label phase 1b (NCT02203773) and phase 1/2 (NCT02287233) clinical trials assessing the safety and efficacy of venetoclax in combination with decitabine or azacitidine, and LDAC, respectively. Patients had newly diagnosed AML and were ineligible for intensive chemotherapy due to comorbidities or age. Patients in the venetoclax plus HMA trial were treated with 400 mg of venetoclax daily and coadministered either 20 mg/m2 of decitabine on days 1-5 or 75 mg/m2 of azacitidine on days 1-7 within each 28-day cycle. Patients in the venetoclax plus LDAC trial received 600 mg of venetoclax daily, with 20 mg/m2 of LDAC on days 1-10 of each cycle. Treatment continued until disease progression or unresolved toxicities. Disease response was first assessed via bone marrow at the end of cycle 1. Patients with resistant disease received therapy cycles without delay; those who achieved a response of morphologic leukemia free state (MLFS) or complete remission with incomplete marrow recovery (CRi) could delay initiation of the subsequent cycle to allow blood count recovery for up to 14 days, or until ANC was ≥500 cells/mL. Patients with drug-related Grade 4 neutropenia for more than 1 week during subsequent cycles had dose delay until ANC rose to ≥500 cells/mL. After cycle 3, patients who achieved complete remission (CR) or CRi who continued to require delay of treatment due to cytopenias, had venetoclax administered for only 21 days per cycle. In this combined analysis, the safety and management of neutropenia are evaluated, including absolute neutrophil counts (ANC) over time, treatment delays, and rate of granulocyte colony stimulating factor (GCSF) administration (based on institutional practice).
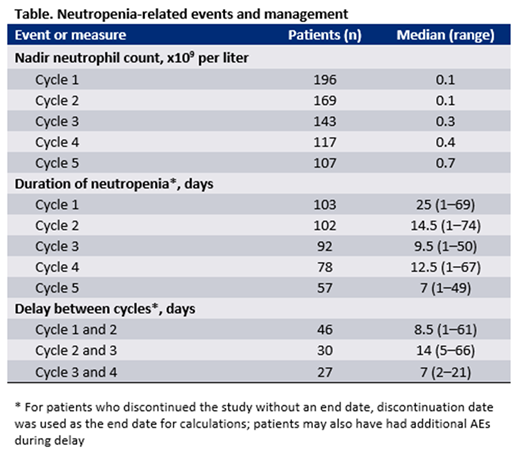
RESULTS: Data cutoffs were August 31st and January 30th, 2018 for the HMA and LDAC studies, respectively. All patients treated at the label-recommended dose of venetoclax (400 mg in combination with HMAs and 600 mg in combination with LDAC) were analyzed as a single group, as neutropenia management was similar regardless of combination treatment. Of 197 venetoclax-treated patients analyzed, 84 were coadministered azacitidine, 31 received decitabine, and 82 received LDAC. The median age of all patients was 74 (range: 61-90) and 36% had secondary AML. Median time on treatment was 5.4 months (range: 0.1-37), and the median time to first response was 1.2 months (range: 0.7-15). Rates of CR and CRi were 38% and 26% (CR + CRi: 64%), respectively. Grade ≥3 ANC-related AEs were: febrile neutropenia (45%) and neutropenia (21%). Key neutropenia related outcomes are shown in the Table. Overall, 48% of patients had per protocol dose delays after confirmation of morphologic marrow response due to neutropenia, and the median time between cycles 1 and 2 of therapy was 8.5 days (1-61). Forty-three percent of patients had treatment delays after cycle 1, and 2% had treatment discontinuations due to febrile neutropenia or grade ≥3 neutropenia. ANC was lowest during cycle 1 combination therapy (median 0.1 ´109 /L), and was less severe over time with continuation of treatment. Thirty-seven percent of patients were administered concomitant GCSF for supportive care. Detailed supportive care and management analysis, including respective outcomes and timing of neutrophil recovery, will be updated for the presentation.
CONCLUSIONS: Neutropenia in patients with AML, treated with venetoclax in combination with either azacitidine/decitabine or LDAC, was a common occurrence; however, ANC was successfully prevented and managed in most patients with a combination of close monitoring, delay between cycles and duration reduction for patients with leukemia-free bone marrow.
Pratz:Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium/Takeda: Research Funding. Wei:Janssen: Honoraria; Macrogenics: Honoraria; Walter and Eliza Hall Institute: Other: former employee, Patents & Royalties: receives a fraction of its royalty stream related to venetoclax; Amgen: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Genentech: Honoraria; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astellas: former employee, Honoraria; Pfizer: Honoraria; Servier: Honoraria, Research Funding. Pollyea:Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forty-Seven: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Diachii Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees. Jonas:AbbVie, Amgen, GlycoMimetics: Other: Travel expenses; AbbVie, Amgen, Celgene, GlycoMimetics, Jazz, Pharmacyclics, Tolero: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Accelerated Medical Diagnostics, AROG, Celgene, Daiichi Sankyo, Esanex, Forma, Genentech/Roche, GlycoMimetics, Incyte, LP Therapeutics, Pharmacyclics: Research Funding. Fiedler:Amgen, Pfizer, Novartis, Jazz Pharmaceuticals, Ariad/Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Amgen, Jazz Pharmaceuticals, Daiichi Sanchyo Oncology, Servier: Other: Support for meeting attendance; Amgen, Pfizer, Abbvie: Other: Support in medical writing. Recher:Macrogenics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria; Sunesis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hong:Genentech Inc.: Employment, Equity Ownership; Roche: Equity Ownership. Potluri:AbbVie, Inc.: Employment, Other: Stock/stock options. Miller:AbbVie: Employment, Other: stock or options. Roboz:Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal