Introduction:
Acute myeloid leukemia (AML) is a heterogeneous disease with varied outcomes dependent on patient cytogenetic and mutational status. Thirty percent of adults with newly diagnosed AML have a mutation in the fms-related tyrosine kinase 3 (FLT3) gene. Midostaurin is a small molecule inhibitor that acts on multiple receptor tyrosine kinases, including FLT3. The RATIFY trial showed improved overall survival (OS) and event-free survival in patients treated with daunorubicin and cytarabine (7+3) plus midostaurin (Stone et al, NEJM 2017). In this trial, a dose of daunorubicin 60 mg/m2 was administered. High dose (HD) 90 mg/m2 daunorubicin significantly improved the rate of complete remission and overall survival, including in patients with FLT3-ITD (Luskin et al, Blood 2016). HD daunorubicin has also been shown to be more effective than idarubicin in patients with FLT3-ITD AML (Lee et al, J Clin Oncol 2017). This data raises the question of whether the combination of midostaurin and HD daunorubicin would further improve outcomes of FLT3 mutated AML patients, while maintaining a tolerable safety profile. The objective of this study is to describe the safety and efficacy endpoints of FLT3 mutated AML patients treated with HD daunorubicin plus midostaurin as part of induction therapy.
Methods:
We retrospectively reviewed clinical and molecular data of patients at Memorial Healthcare System, Moffitt Cancer Center, and Sylvester Cancer Center with newly diagnosed FLT3 mutated AML treated from May 1st, 2017 to July 1st, 2019. Clinical data was abstracted in accordance with institutional review board approved protocol. All patients were induced with HD daunorubicin 90 mg/m2 on days 1-3, cytarabine 100 mg/m2 on days 1-7, and midostaurin 50 mg PO twice daily on days 8-21. Growth factor and antimicrobial support were used per institutional guidelines. Demographics were analyzed using descriptive statistics. OS was analyzed using Kaplan Meier method. Other efficacy outcomes were CR, CRi (assessed according to the European Leukemia Network Criteria for AML), proportion of patients needing re-induction, and proportion of patients who underwent hematopoietic stem cell transplant (HSCT). Safety outcomes were adverse events (AEs) and early (30- and 60-day) mortality.
Results:
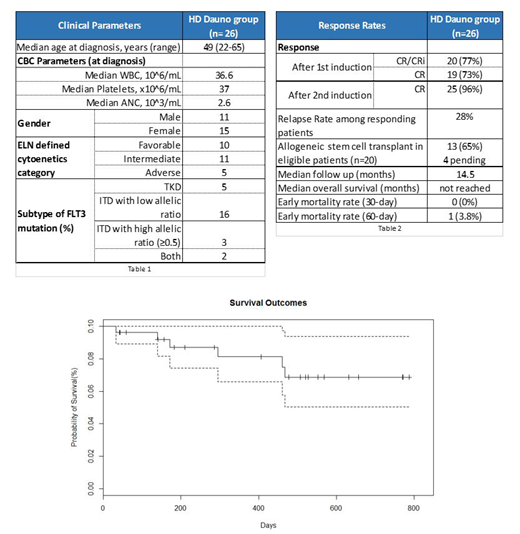
Twenty-six patients were included in the final analysis. Patient characteristics are outlined in TABLE 1. All patients were FLT3 mutated, as confirmed with molecular studies. The FLT3 subtype was ITD (high) in 3 patients, ITD (low) in 16 patients, TKD in 5 patients, and both in 2 patients. Seventy-seven percent of patients achieved a CR/CRi after one induction cycle, and 96.2% attained CR after two induction cycles. Median time to ANC and platelet recovery was 28 and 26 days, respectively. One patient died during the first 60 days, due to Enterococcus sepsis. The most common non-hematological AEs were nausea (77%), diarrhea (62%), mucositis (58%), rash (54%), and increased ALT (54%). Cumulative incidence of relapse in the cohort was 28% (n=7). Four patients relapsed pre-transplant and achieved CR2 with additional therapy. All 7 of these patients had co-occurring mutations of various types. Of the 20 patients who were considered transplant eligible, 13 (65%) underwent HSCT and 4 (20%) are pending transplant. Of the 13 transplanted patients, 3 experienced relapse post-transplant. After a median follow up of 14.5 months, median OS has not been reached.
Conclusion:
In our multi-center experience, induction with HD daunorubicin, cytarabine, and midostaurin is clinically effective and seems to be well tolerated. Short term mortality was low and AEs were manageable, with no unexpected safety signals. Also, CR/CRi rates were higher than previously reported, suggesting that the combination of HD daunorubicin and midostaurin may improve the outcomes of patients with FLT3 mutated AML. Future analyses with larger patient samples and longer follow up are warranted to further evaluate long-term safety and efficacy for this regimen.
Sandoval-Sus:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Bradley:AbbVie: Other: Advisory Board. Talati:Agios: Honoraria; Celgene: Honoraria; Pfizer: Honoraria; Astellas: Honoraria, Speakers Bureau; Daiichi-Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria, Speakers Bureau. Watts:Pfizer: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Sallman:Abbvie: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Research Funding; Incyte: Speakers Bureau; Celyad: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding, Speakers Bureau. Sweet:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau; Jazz: Speakers Bureau; Incyte: Research Funding; Pfizer: Consultancy; Stemline: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees. Lancet:Daiichi Sankyo: Consultancy, Other: fees for non-CME/CE services ; Agios, Biopath, Biosight, Boehringer Inglheim, Celator, Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm, Novartis: Consultancy; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal