Introduction: CD20-TCB (RG6026) is a novel T-cell-engaging bispecific (TCB) antibody with a '2:1' molecular format that comprises two fragment antigen binding regions that bind CD20 (on the surface of B cells) and one that binds CD3 (on the surface of T cells). CD20-TCB offers the potential for increased tumor antigen avidity, rapid T-cell activation, and enhanced tumor cell killing versus other bispecific formats. An ongoing Phase I dose-escalation study (NP30179; NCT03075696) has shown promising antitumor activity and acceptable safety in relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL) patients (pts) (Dickinson et al. ICML 2019). We investigated population pharmacokinetics (popPK) and exposure-response (E-R) relationships for CD20-TCB in NP30179.
Methods: Indolent (i) and aggressive (a) R/R NHL pts received CD20-TCB doses of 0.005 to 25mg every 2 or 3 weeks following single 1000mg obinutuzumab (G) pre-treatment (Gpt) on Cycle 1 Day −7 to mitigate for cytokine release syndrome (CRS). Serial and spare PK data collected from pts were used to develop a popPK model in NONMEM v7.4. Physiologically relevant covariates were investigated for their potential influence on CD20-TCB PK variability. Using the previously established G popPK model (Gibiansky et al. CPT Pharmacometrics Syst Pharmacol 2014;3:e144), full G concentration-time profiles were constructed in order to estimate CD20-TCB receptor occupancy (RO%) in the presence of G concentrations competing for CD20 receptors over time. E-R relationships between CD20-TCB time-averaged RO% (AvgRO%) up to Cycle 3 Day 1 and objective response rate (ORR) and complete response rate (CRR) were investigated in aNHL pts who reached Cycle 3 Day 1, and the relationship between CD20-TCB AvgRO% over the first 24 hours (as the majority of events occurred within the first 24 hours) and CRS, the most common safety event, as defined by Lee et al. (Blood 2014;124:188-95), was investigated in iNHL and aNHL pts combined using logistic regression.
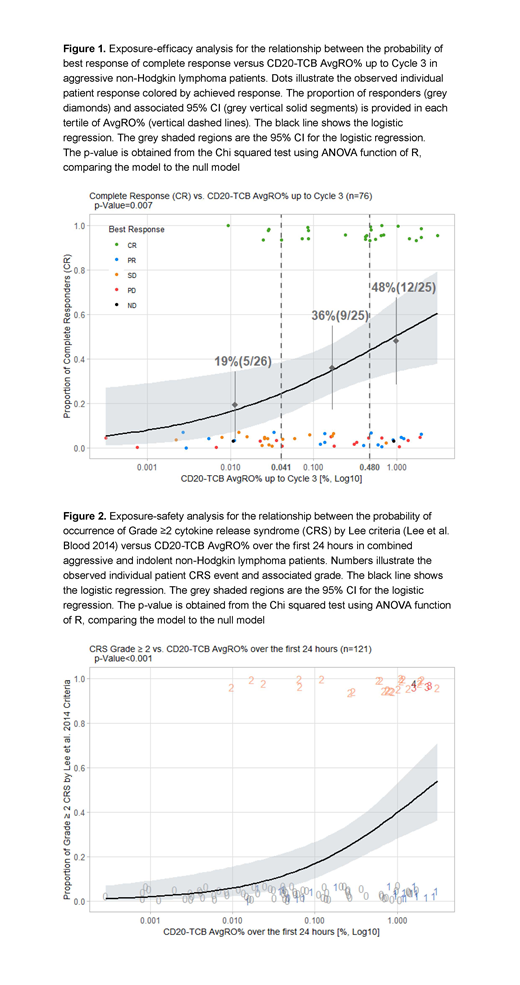
Results: The popPK analysis included 139 iNHL and aNHL pts with at least one PK sample. The E-R analysis for efficacy included 76 aNHL pts with PK and efficacy data at Cycle 3 Day 1. The E-R analysis for safety included 121 iNHL and aNHL pts with PK and safety data. CD20-TCB PK was best described using a two-compartment PK model with linear clearance. Body weight had a statistically significant influence on PK and was retained using theory-based allometric scaling. There were no obvious differences in PK between iNHL and aNHL pts. In aNHL pts, logistic regression analyses demonstrated a significant positive E-R relationship between AvgRO% up to Cycle 3 Day 1 and efficacy (ORR and CRR, p=0.007; Figure 1 for CRR). In the highest tertile of AvgRO% (≥0.48%), the observed ORR was 76% versus 38.5% (<0.041%) and 52% (0.041%-0.48%) in the lower tertiles; CRR was 48% versus 19.2% and 36%. A significant positive E-R relationship was identified between CD20-TCB AvgRO% over the first 24 hours and Grade (Gr) ≥2 CRS (p<0.001; Figure 2), consistent with the clinical safety profile showing first-dose-dependent CRS, with the majority of events occurring within the first 24 hours (Dickinson et al. ICML 2019). Following administration of a CD20-TCB dosing regimen of 10/16mg q3w (10mg in Cycle 1 followed by 16mg thereafter), the AvgRO% (median [P10-P90]) up to Cycle 3 was 0.466% (0.249%-1.63%), corresponding to an anticipated CRR at Cycle 3 of 43.6% (38.2%-54.7%) based on the exposure-efficacy model (Figure 1), in line with the clinical CRR seen in the 10 and 16mg q3w dose cohorts previously reported (Dickinson et al. ICML 2019). The resulting AvgRO% over the first 24 hours following an initial 10mg dose in Cycle 1 was 0.797% (0.344%-1.78%), corresponding to a Gr ≥2 CRS rate of 36.7% (27.1%-45.4%) based on the exposure-safety model (Figure 2), in line with the CRS incidences observed in the 10mg q3w dose cohort reported previously (Dickinson et al. ICML 2019). These PopPK and ER analyses are being used to simulate CD20-TCB dosing regimens that maximize efficacy and mitigate CRS risk, including fixed and step-up dosing regimens.
Conclusions: PopPK and E-R relationships are characterized for the novel CD20/CD3 TCB antibody CD20-TCB and are being used to support optimal biological-dose selection as a single agent in ongoing combination investigations (Morschhauser et al. ASH 2019; Hutchings et al. ASH 2019).
Djebli:F. Hoffmann-La Roche Ltd: Employment. Jaminion:F. Hoffmann-La Roche Ltd: Employment. Laurent:F. Hoffmann-La Roche Ltd: Employment. Mercier:F. Hoffmann-La Roche Ltd: Employment. Kratochwil:F. Hoffmann-La Roche Ltd: Employment. Bröske:Roche: Employment, Equity Ownership. Dimier:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Ferlini:Roche: Employment, Equity Ownership. Moore:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Weisser:Pharma Research and Early Development Roche Innovation Center Munich: Employment, Equity Ownership, Patents & Royalties. Morcos:Roche: Employment, Equity Ownership.
CD20-TCB (also known as RG6026, RO7082859) is a full-length, fully humanized, immunoglobulin G1 (IgG1), T-cell-engaging bispecific antibody with two fragment antigen binding ('Fab') regions that bind to CD20 (on the surface of B cells) and one that binds to CD3 (on the surface of T cells) (2:1 format). The 2:1 molecular format of CD20-TCB, which incorporates bivalent binding to CD20 on B cells and monovalent binding to CD3 on T cells, redirects endogenous non-specific T cells to engage and eliminate malignant B cells. CD20-TCB is an investigational agent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal