Background and Rationale: T-cell large granular lymphocytic leukemia (T-LGLL) is an incurable clonal proliferation of CD8+ memory T-cells that leads to profound neutropenia and anemia with limited treatment options. The primary driver of T-LGLL is overexpression of interleukin-15 (IL-15), a gamma-chain cytokine. Previously, we have demonstrated that mice overexpressing IL-15 develop DNA hypermethylation and chromosomal instability that leads to the spontaneous development of LGLL (Mishra et al. Cancer Cell 2012). Further, the IL-15 promoter is known to be hypermethylated in cutaneous T-cell lymphoma (CTCL), another IL-15 driven malignancy (Mishra et al. Cancer Discovery 2016). In CTCL patients, the counterintuitive increase in IL-15 mRNA was due to hypermethylation of its promoter at the repressor binding sequences in the IL-15 gene. However, the methylation status of the IL-15 promoter in T-LGLL patients remains unknown.
Concept: We hypothesize that the IL-15 promoter is hypermethylated in patients with T-LGLL, leading to aberrant overexpression of IL-15 and that this hypermethylation is a critical event in the leukemogenesis of T-LGLL. If true, demethylation of the IL-15 promoter with a resultant decrease in IL-15 transcripts should lead to apoptosis of T-LGLL cells. Hypomethylation of the IL-15 promoter, therefore, may provide a novel therapeutic approach to inhibiting IL-15, the primary driver of T-LGLL.
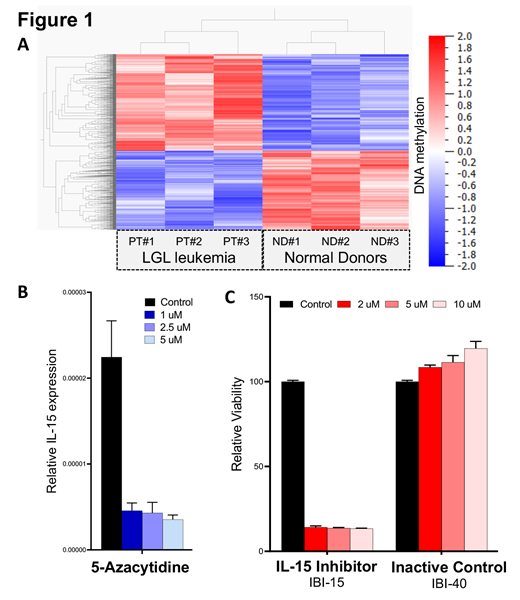
Results: CD3+/CD8+/CD5-/dim T-cells were purified from peripheral blood of LGLL patient (n=3) and normal donor (ND) (n=3) by flow cytometry sorting. We analyzed DNA methylation and gene expression profiling using reduced representation bisulfite and RNA sequencing. With bioinformatics analysis, we determined differential methylation (1-way ANOVA P= 0.0178) and expression (1-way ANOVA P =0.0059). These data sets revealed significant differential hypermethylation of gene promoters in leukemic samples, compared to controls (Figure 1A). Reduced representation bisulfite sequencing that can identify differentially methylated regions at single base-pair resolutions demonstrated an increase in DNA methylation of the IL-15 promoter in patient samples over controls. To determine the functional significance of this finding, we treated the MOTN-1 T-LGLL cell line in vitro with the hypomethylating agent, 5-azacytidine (5-aza) at concentrations of 0.5 uM, 1 uM, 2.5 uM, and 5 uM. At 24 and 48 hours, a marked decrease in the viability of T-LGLL cells was observed, from 100% to 49.50%, p=0.037; particularly at higher concentrations of 5-aza (100% to 27% +11.30%, p=0.0030). Next, we sought to determine whether 5-aza induced hypomethylation of the IL-15 promoter. IL-15 gene expression in MOTN-1 T-LGLL cells treated with 5-aza was measured in comparison to control treated MOTN-1 cells. A marked decrease in IL-15 expression was observed at all concentrations of 5-aza compared to control (Figure 1B, p=0.0001). These results confirm that 5-aza leads to decreased transcription of the IL-15 gene, possibly due to hypomethylation of the IL-15 promoter. Finally, to determine whether a decrease in IL-15 alone was the cause of increased apoptosis of T-LGLL cells, we exposed MOTN-1 cells to a novel IL-15 inhibitor, IBI-15, and compared cell viability against MOTN-1 cells exposed to an inactive control, IBI-40. Even more profound decrease in cell viability was observed utilizing IBI-15 that targets the binding of IL-15 to its receptor (Figure 1C). Together, these data suggest that hypermethylation of the IL-15 promoter is critical to the pathogenesis of T-LGLL, and that treatment with 5-aza is sufficient to induce hypomethylation of the IL-15 promoter, decrease IL-15 transcription, and induce apoptosis in T-LGLL cells.
Conclusions: Hypermethylation of the IL-15 promoter, with subsequent increase in IL-15, is critical to the pathogenesis of T-LGLL. Inhibition of the IL-15 promoter hypermethylation by 5-aza leads to down-regulation of the IL-15 gene transcript, which is sufficient to induce apoptosis of T-LGLL cells. These data suggest that 5-aza induced hypomethylation may be a novel method to induce IL-15 inhibition and a potentially efficacious clinical strategy against T-LGLL.
Brammer:Bioniz Therapeutics, Inc.: Research Funding; Viracta Therapeutics, Inc.: Research Funding; Verastem, Inc: Research Funding. Porcu:Daiichi: Research Funding; BeiGene: Other: Scientific Board, Research Funding; Spectrum: Consultancy; Viracta: Honoraria, Other: Scientific Board, Research Funding; Innate Pharma: Honoraria, Other: Scientific Board, Research Funding; Kyowa: Honoraria, Other: Scientific Board, Research Funding; ADCT: Research Funding; Incyte: Research Funding.
IBI-15 IBI-40 IL-15 inhibitor
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal