Introduction
Telomere length is shortened in patients with idiopathic aplastic anemia (AA) and other bone marrow failure disorders (BMFD) and predicts risk of clonal evolution (CE), relapse and overall survival (OS). Telomereopathies predominantly cause bone marrow failure, are multi-systemic disorders with variable penetrance, and may involve inter-play of other factors in disease manifestation and organ affliction. Telomere length (TL) in AA and other hypocellular BMFD, independent of mutations in telomere genes (TGC), has not been studied as a scoring tool, as well predicting the risk of affliction of other organ/systems in these disorders. We systematically review a large cohort of 472 patients in a single centre with AA/BMFD using TL and TGC analysis as a discriminator, to study risk of CE and OS, manifesting with liver/lung and skin complications, cancer predisposition and likelihood of a family member presenting with cytopenias.
Methods
We screened 1060 consecutive patients at a single centre from the years 2011-18, with AA or unexplained cytopenias for telomere length (TL) analysis using a multiplex qPCR methodology as described by Cawthon et al. 472 (44.5%) patients had TL less than the 25th centile, of whom 243 had <1st centile, 122 had <10th and 107 had TL between the 10-25th centile. These 472 patients underwent TGC mutation analysis on a customised panel of 12 TGC genes (TERT, TERC, DKC1, TINF2, NHP2, NOP10, RTEL1, CTC1, USB1, WRAP53, ACD and PARN) using deeply parallel sequencing and were studied alongside their clinical parameters, disease progression, treatments and OS.
Results
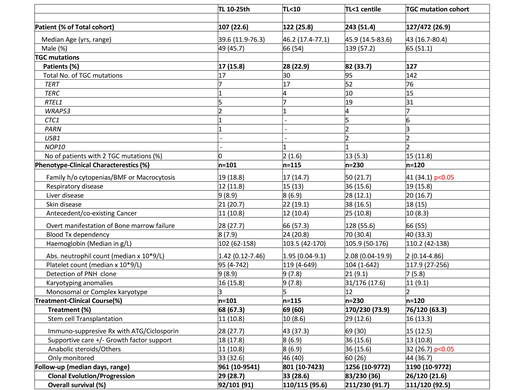
Table attached. The median age and gender across the 3 cohorts studied (TL 10-25th centile, <10th centile and <1st centile) were comparable, with a median age of 43 for the entire cohort. 127 (26.9%) of patients were detected to have a total of 142 mutation in the TGC.
A third of patients with BMFD and TL<1st centile had a TGC mutation, but the prevalence did not statistically differ with the other cohorts of TL <10th centile and between 10-25th centile. Patients with TL<1st centile can have mutations in 2 telomere genes, but this is less frequently seen when TL<10th centile and not seen in the cohort with TL between 10-25th centile.
TL does not correlate with the manifestation and presence of liver, respiratory and skin problems of telomere disease. TL in BMFD does not associate with increased predisposition to cancer, as their presence was not statistically significant across the 3 groups.
Disease manifestation with bone marrow failure, haematological indices, need for blood transfusions, presence of a PNH clone and karyotypic abnormalities, including complex and monosomal karyotype were not dissimilar across the 3 TL cohorts.
Not surprisingly, there were more family members with features of cytopenias and BMFD, on screening of patients with a confirmed TGC mutation.
TL did not predict a difference in treatment strategies used across the 3 groups, although more patients with a TGC mutation received anabolic steroids.
The risk of clonal evolution to PNH or MDS/AML and overall survival was again similar across the 3 TL cohorts with an OS of 93% at 1078 days follow-up for the entire cohort.
Conclusion
TL can reliably be used as a screening tool to investigate patients for further TGC mutation analysis in patients with AA or BMFD. Heterozygous state mutations in TERT are the commonest, and can be associated with mutations in other TGC genes, particularly RTEL1 and TERC. This may cause a compounding factor in shortening the TL further. However, TL<1st centile does not associate with more severe cytopenias (BMFD) or predict more multi-system manifestation or increased cancer pre-disposition. Indeed, in our cohort it failed to show an increased risk of clonal evolution or decreased OS. Telomere biology is a dynamic process and assessments at different time points, from diagnosis to the time point of progression and transformation, may yield better understanding in pathogenesis of BMFD.
TL and TGC mutation analysis in a single centre study from 2011-18.
Kizilors:Incyte biosciences: Research Funding. Mufti:Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal