
Introduction: Complement C5-inhibitor eculizumab is a current standard of care in patients with paroxysmal nocturnal hemoglobinuria (PNH). Elizaria® is the first registered biosimilar of the reference medicinal product Soliris®.
Purpose: To demonstrate Elizaria® comparative efficacy and safety vs. reference product Soliris® in patients with paroxysmal nocturnal hemoglobinuria.
Methods: 32 patients with PNH were stratified based on the previous eculizumab treatment status (eculizumab-naive patients/patients treated with eculizumab at a maintenance regimen before enrollment) and randomized to receive Elizaria® (n = 16) or Soliris® (n = 16). Eculizumab-naive patients with baseline LDH level ≥1.5 times the upper limit of normal started induction phase of four weekly infusion of the study medications at dose of 600 mg, with subsequent maintenance therapy at dose of 900 mg every two weeks. Previously treated patients started the study treatment at the maintenance dose. The total treatment duration was 26 weeks. Comparative evaluation of chronic hemolysis based on the area under the curve "LDH concentration-time" (LDH AUC) during the maintenance therapy period was the primary endpoint of the study. The main secondary efficacy parameters were hemoglobin dynamics, breakthrough hemolysis and transfusion dependence. PK profile as well as through eculizumab and membrane attack complex (MAC) concentration were also assessed during the treatment.
Results: Thirty patients have completed the study without significant protocol deviations (16 patients in Elizaria® group and 14 patients in Soliris® group).
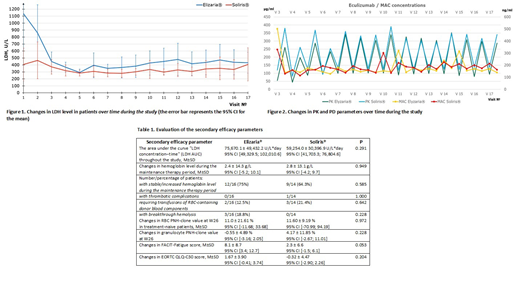
The LDH AUC was highly variable between the patients but the means were not statistically differ between the treatment groups: 62,957.6 ± 46,066.5 U/L*day (95% CI [38,410.4; 87,504.7]) and 49,702.6 ± 26,182.1 U/L*day (95% CI [34,585.5; 64,819.7]) in Elizaria® and Soliris® groups respectively. Point estimation of the intergroup difference in LDH AUC of 13255,0 U/L*days (95% CI [-10492,9; 37002,8]) comprises 12,3% of the pre-specified non-inferiority margin of 150,635 U/L*days, that is not supposed clinically significant difference (Figure 1).
The treatment groups were also comparable on all the secondary efficacy parameters (Table 1).
The comparative PK parameters of eculizumab and MAC concentration have been demonstrated in the treatment groups in all studied time points. Thus, the median elimination half-life (T1/2) of eculizumab amounted to 187,7 h (IQR 165,7) in Elizaria® group and to 282,0 h (IQR 152,3) in Soliris® group (p = 0.236). The minimum concentration (Cmin) of eculizumab amounted to (64.89 ± 25.96) µg/mL in Elizaria® group and to (94.13 ± 58.76) µg/mL in Soliris® group (p = 0.065). The median residence time (MRT) of eculizumab amounted to 269,5 h (IQR 257.3) in Elizaria® group and to 393,1 h (IQR 240,9) in Soliris® group (p = 0.206). By the end of the study, the mean MAC values one hour after the study products infusion amounted to (170.41 ± 72.21) ng/mL and (214.08 ± 93.57) ng/mL in Elizaria® and Soliris® groups, respectively (Figure 2).
Both Elizaria® and Soliris® demonstrated the similar safety profile. Thirteen adverse drug reactions (ADR) in 5 patients were reported in the study: 9 - in 3 Elizaria® patients, whereas 4 were reported in 2 Soliris® patients. ADRs were observed in the following system organ classes: Investigations (9.4%), Blood and lymphatic system disorders (6.3%), Infections and infestations (6.3%), Renal and urinary disorders (3.1%), General disorders and administration site conditions (3.1%), Metabolism and nutrition disorders (3.1%).
No new cases of anti-drug antibody formation have been revealed during the study.
Conclusion: Thus, the results of the clinical trial confirm that proposed biosimilar Elizaria® is comparable to the reference product Soliris® in term of efficacy, safety, immunogenicity and PK/PD parameters in the treatment of paroxysmal nocturnal hemoglobinuria.
Kulagin:Alexion Pharmaceuticals, Inc:: Consultancy, Honoraria; JSC GENERIUM: Consultancy, Honoraria, Research Funding. Ptushkin:Alexion Pharmaceuticals, Inc:: Consultancy, Research Funding; JSC GENERIUM: Research Funding; Janssen: Consultancy; AbbVie: Consultancy; Roche: Consultancy. Lukina:JSC GENERIUM: Research Funding; Sanofi Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Reimbursement, Research Funding; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Reimbursement. Gapchenko:JSC GENERIUM: Employment. Markova:JSC GENERIUM: Employment. Zuev:JSC GENERIUM: Employment. Kudlay:JSC GENERIUM: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal