
Crovalimab (RO7112689) is a novel anti-human C5 antibody engineered with Sequential Monoclonal Antibody Recycling Technology (SMART Ig)(Fukuzawa et al., Sci Rep. (2017) 7(1):1080), resulting in significant half-life extension and enabling infrequent SC dosing using small volumes (1mL - 4mL) in C5 mediated diseases. We aimed at characterizing the exposure-response relationship of crovalimab used to define the minimum concentration of crovalimab achieving complete terminal inhibition.
To establish pharmacokinetics (PK), pharmacodynamics (PD), safety, efficacy, and optimal dose of crovalimab, we conducted a four-part adaptive clinical trial (Figure 1):
Part 1: 15 healthy subjects were enrolled. 3 received 75 mg RO7112689 IV, 3 received 125 mg RO7112689 IV, 3 received 100 mg RO7112689 SC, and 6 received placebo
Part 2: 10 treatment-naïve PNH patients were enrolled in Part 2 to receive increasing IV doses of 375mg, 500mg, and 1000mg on days 1, 8 and 22, respectively, followed by weekly doses of 170mg SC starting on day 36
Part 3: 19 eculizumab pre-treated PNH patients were enrolled in Part 3 to receive 1000mg IV before randomization into 3 different arms:
Arm A: 680mg SC Q4W (N=7)
Arm B: 340mg SC Q2W (N=6)
Arm C: 170mg SC QW (N=6)
SC dosing was initiated on day 8 after the IV dose in all the dosing groups.
Part 4: 5 eculizumab pre-treated PNH patients and 5 treatment-naïve PNH patients are planned to be enrolled to receive IV dose of 1000mg on Day 1 followed by SC dose of 340mg on Day 2, Day 8, Day 15, Day 22 followed by SC dose of 680mg given Q4W from Day 29
Crovalimab concentrations and free C5 were measured using a validated ELISA. A population PK model was developed using all the available data to describe the crovalimab concentration-time profiles. Crovalimab PD was assessed by evaluating the extent and duration of terminal complement inhibition, quantified using a validated, functional ex vivo liposome immunoassay (LIA) (http://www.wakodiagnostics.com/r_ch50.html). Relationships between crovalimab PK and PD were analyzed using graphical analysis.
The PK was best described by a two-compartment open model with first-order elimination and absorption. To describe the PK in eculizumab pre-treated patients, elimination of crovalimab was modeled as a combination of the first-order elimination used for naïve patients and a faster clearance which decreases exponentially over time. Body weight was introduced using allometry on the clearance and volume of the distribution. After SC administration, bioavailability is estimated at 100% and terminal half-life around 30 days.
In all PNH patients, complete complement inhibition (defined as LIA <10 U/ml, the LLOQ of the assay) was achieved immediately after end of infusion following the initial dose and maintained across the different dosing intervals investigated in the majority of the patients (Figure 2). Complete inhibition of free C5 (defined as free C5 <0.05μg/mL) was also achieved after end of infusion and maintained throughout the dosing intervals reflecting the LIA profiles.
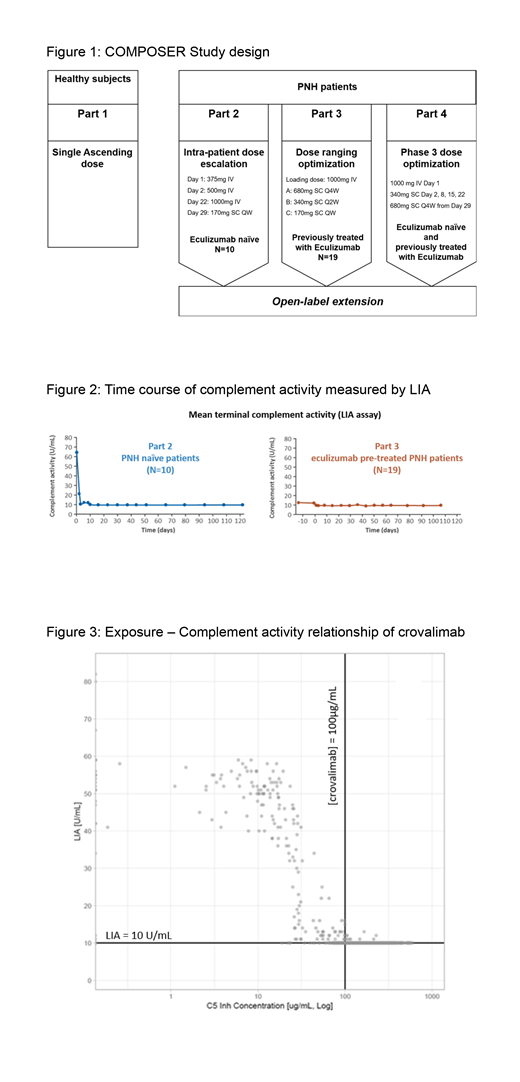
By pooling all the PK and PD data from the 3 parts of the COMPOSER study, crovalimab was shown to induce a concentration-dependent inhibition of serum hemolytic activity and of free C5 which closely correlates with terminal complement inhibition. Collectively, these data indicate that approximately 100μg/mL of crovalimab are required to achieve complement inhibition (Figure 3).
At the target concentration of crovalimab between 80-100ug/mL, patients achieve complete terminal complement inhibition. Data collected in the ongoing Part 4 of COMPOSER will be used to confirm the target concentration of crovalimab. Exposure-response characterization demonstrated the potential of crovalimab as an infrequent, subcutaneous therapy for PNH.
Sostelly:F. Hoffmann-La Roche: Employment. Buatois:F. Hoffmann-La Roche: Employment. Soubret:F. Hoffmann-La Roche: Employment, Equity Ownership. Klughammer:F. Hoffmann-La Roche Ag: Employment, Equity Ownership. Hsu:Roche/Genentech: Employment, Equity Ownership. Jordan:Roche Diagnostics GmbH: Employment; F. Hoffmann-La Roche: Equity Ownership. Bucher:F. Hoffmann-La Roche: Employment. Charoin:F. Hoffmann-La Roche: Employment. Gotanda:Chugai Pharmaceutical Co., Ltd.: Employment. Shinomiya:Chugai Pharmaceutical Co., Ltd.: Employment, Patents & Royalties: (WO2018143266) A PHARMACEUTICAL COMPOSITION FOR USE IN THE TREATMENT OR PREVENTION OF A C5-RELATED DISEASE AND A METHOD FOR TREATING OR PREVENTING A C5-RELATED DISEASE. Nagy:Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Panse:Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Chugai: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees. Yoon:Genentech, Inc.: Research Funding; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; MSD: Consultancy; Kyowa Hako Kirin: Research Funding; Yuhan Pharma: Research Funding; Janssen: Consultancy. Peffault de Latour:Novartis: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Alexion: Consultancy, Honoraria, Research Funding. Nishimura:Alexion: Honoraria, Research Funding; Chugai: Consultancy, Membership on an entity's Board of Directors or advisory committees. Röth:Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Apellis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal