Increasing evidence suggests that bone marrow microenvironment act as a sanctuary site for acute myeloid leukemia (AML) cells and provides protection from conventional chemotherapy agents. Recently, extracellular vesicles (EVs) have attracted substantial attention as a carrier of complex intercellular information by transferring microRNA, mRNA and proteins. We undertook a study to delineate the molecular mediators and potential role of extracellular vesicles in stromal microenvironment mediated drug resistance in AML.
We performed a series of in vitro experiments with AML cell lines (U937, THP-1, Kasumi-1) and primary cells to evaluate their response to daunorubicin (DNR) and cytarabine (AraC) with stromal cells (HS-5 cell line). Towards this we co-cultured the leukemic cells with stromal cells in a contact dependent and contact independent (transwell plates) system and with EVs derived from HS-5 culture media using well established methods (Suzanne et al, Blood 2015). The percentage of viability was calculated using Annexin V/7AAD staining by flow cytometry. Gene expression profiling was done using Agilent Human Whole Genome 8x60K Gene Expression Array. The quantification of extracellular vesicle was performed using NanoSight LM10.
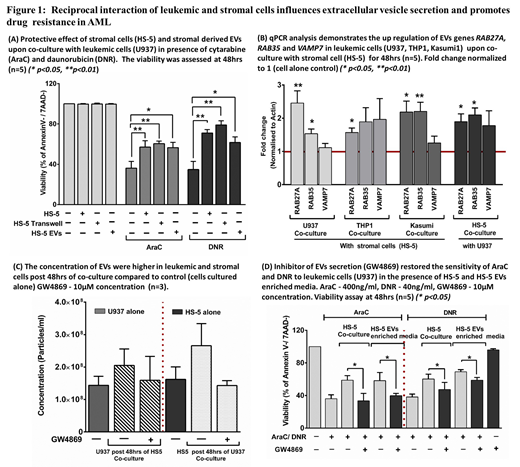
Direct stromal co-culture experiments with AML cells demonstrated a significant stromal cell mediated protective effect against AraC and DNR in cell lines (figure 1A) and primary cells [AraC p < 0.01; DNR p < 0.001 (n=50)]. A similar significant protective effect was also seen in contact independent system and EVs alone treated leukemic cells (supplemented in place of HS-5 co-culture). Gene expression profiling analysis of leukemic cells (U937) and stromal cell (HS-5) post co-culture revealed a bidirectional enrichment of genes involved in extracellular vesicle biogenesis and secretion (p < 0.001) along with a significant dysregulation of PI3K-AKT signaling in leukemic cells. We have previously reported that stromal EVs activates PI3K-AKT signaling and mediates drug resistance in leukemic cells similar to direct stromal co-culture (Blood 2017 130:1160). In addition to PI3K-AKT signaling, our qPCR validation also confirmed the significant up regulation of genes which are involved in EVs secretion (RAB27A, RAB35 and VAMP7) in leukemic cells as well as stromal cells post co-culture (figure 1B). Hence, we quantified the amount of EVs production in leukemic and stromal cells post 48hrs of co-culture where the number of EVs showed a trend towards increase in co-cultured leukemic and stromal cells when compared to the cells cultured alone (figure 1C). We also noted that treatment with neutral sphingomyelinease inhibitor GW4869 a known inhibitor of EVs secretion was able overcome the stroma mediated drug resistance significantly in leukemic cell lines (figure 1D) and also in primary AML cells [AraC p < 0.01; DNR p < 0.001 (n=6)].
Our results illustrate that reciprocal interaction of leukemic and stromal cells influences the secretion of extracellular vesicles and plays a significant role in mediating drug resistance. We further demonstrated that inhibiting extracellular vesicles secretion was able to overcome the stromal microenvironment mediated drug resistance in AML illustrating a potentially novel therapeutic strategy. Additional studies are required to explore and characterize the cargo (microRNA and proteins) in detail of these EVs and the mechanism/s by which they mediate drug resistance.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal