BACKGROUND
Non Valvular Atrial Fibrillation (NVAF) is the most common cardiac arrhythmia among patients with cancer. Anticoagulation in this setting is associated with a higher rate of clinically relevant major and non-major bleeding and therefore, can be especially challenging. Due to concerns about drug interactions in patients receiving chemo-immunoterapy, Low Molecular Weight Heparin (LMWH) has been the most commonly prescribed anticoagulant for stroke prevention as a substitute for vitamin K antagonists. Direct Oral Anticoagulants (DOAC) are an increasing alternative for anticoagulant therapy for stroke prevention in NVAF, but there are are still limited data regarding it's effectiveness and safety for cancer patients receiving active treatment.
AIMS
To assess the effectiveness and safety according to DOAC or LMWH treatment, and to determine the rate of anticoagulant-associated clinically relevant bleeding-free survival in a cohort of cancer patients with NVAF receiving active treatment.
METHODS
From April 2016 to December 2018 we consecutively included NVAF patients with active cancer therapy treated with DOAC or LMWH in a prospective multicenter registry. Patients with prosthetic valves or a life expectancy of less than one month were excluded from this study. Active cancer therapy was defined as evidence of neoplasm with ongoing antineoplastic therapy (chemo-immunotherapy or hormonal treatment). Pharmacological interactions check-up was performed prior election of treatment. Demographic, laboratory, cancer diagnosis, and antineoplastic therapy data were collected. Patients had a minimum follow-up (FU) of 6 months. In patients who received antineoplastic therapy with a potential DOAC interaction, plasma drug concentrations were measured during the FU using the Direct Thrombin Inhibitor Assay from IL (Bedford-MA-USA) for Dabigatran and the Technoclone anti-Xa assay from Technoclone (Vienna-Austria) for Rivaroxaban. Bleeding events were classified according to ISTH criteria.
RESULTS
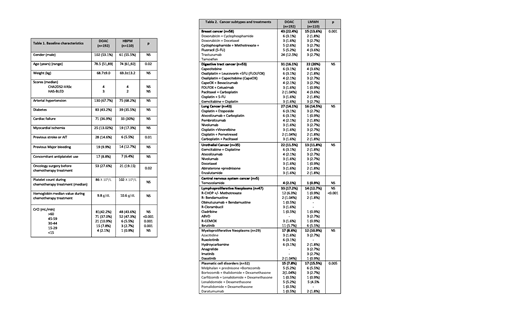
A total of 302 patients with NVAF and active cancer therapy were included. Among all patients, 192 (63.5%) were treated with DOAC (20 dabigatran, 24 rivaroxaban, 80 apixaban and 68 edoxaban) and 110 with LMWH. Mean FU was 14.8 and 12.5 months (DOAC vs LMWH; p:0.53). Demographic characteristics and cancer subtypes and drugs are summarised in table 1 and 2, respectively. In LMWH group, 81.8% (n=90) of patients received full-dose of LMWH, 13.6% (n=15) intermediate dose and only a 4.5% patients received prophylactic doses.
Plasma concentrations were measured in 2 patients receiving dabigatran 110 mg twice daily and enzalutamide. Trough level of our patients was 132.4 and 126.8 ng/mL (12 hours after the last dose). Rivaroxaban plasma samples were collected in 3 patients who received doxorubicin as part of chemotherapy regimen. Plasma rivaroxaban levels, determined 4 hours and 24 hours of the last dose, ranged from 112.4 to 432.3 ng/ml and 49.8 to 216 ng/ml, respectively. Considering these results, DOACs were maintained during antineoplastic treatment.
Stroke or systemic embolism occurred in three patients in the DOAC group (1.04 %/year) and seven patients in the LMWH group (7.2 %/year) [DOAC vs LMWH; p<0.05]. Major bleeding occurred in eight patients in the DOAC group (4.1%/year) and seven patients in the LMWH group (6.5%/year). All reported mortality was disease related. The bleeding-free survival rates were not statistically different between DOAC vs LMWH.
CONCLUSIONS
In our cohort, the stroke and systemic embolism rate was higher in the LMWH group without significant differences in relation to major bleeding events. Further investigations on the optimal management of cancer patients with active therapy and NVAF treated with DOAC are needed. Meanwhile, determining DOAC plasma concentrations could be of profit to personalize anticoagulant therapy for patients with unpredictable drug interactions. Anticoagulation units play a crucial role in offering the best personalised therapy.
Sierra:Novartis: Honoraria, Research Funding, Speakers Bureau; Astellas: Honoraria; Pfizer: Honoraria; Daiichi-Sankyo: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Roche: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal