Thrombin generation and fibrin formation can cause occlusive thrombosis and myocardial infarction is caused by occlusive thrombi. Exposure and release of cardiac myosin (CM) are linked to myocardial infarction, but CM has not been accorded any thrombotic functional significance. Skeletal muscle myosin (SkM), which is structurally similar to CM, was previously shown to exert procoagulant activities (Deguchi H et al, Blood. 2016;128:1870), leading us to undertake new studies of the in vitro and in vivo procoagulant activities of CM.
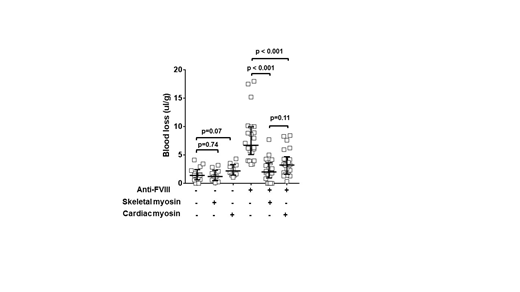
First, the setting of hemophilia A with its remarkable bleeding risk was used to evaluate the procoagulant properties of CM. In studies of human hemophilia plasma and of murine acquired hemophilia A plasma, CM was added to these plasmas and tissue factor (TF)-induced thrombin generation assays were performed. Plasmas included human hemophilia A plasma and C57BL/6J mouse plasma with anti-FVIII antibody (GMA-8015; 5 microgram/mL final). CM showed strong procoagulant effects in human hemophilia A plasma, which is naturally deficient in factor VIII (<1% FVIII). The addition of only CM (12.5-200 nM) greatly increased thrombin generation in a manner comparable to addition of only recombinant FVIII. In the wild-type C57BL/6J mouse plasma, anti-FVIII antibody greatly reduced TF-induced thrombin generation, as reported. When CM (12.5-200 nM) was added to mouse plasma containing anti-FVIII antibodies, TF-induced thrombin generation was concentration-dependently restored. To study the in vivo hemostatic ability of SkM, an acquired hemophilia A mouse model was employed. Intravenous injection of anti-FVIII antibody (GMA-8015; 0.25 mg/kg) or control vehicle was given retro-orbitally to wild type C57BL/6J mice at 2 hours prior to tail cutting. The distal portion of the tail was surgically removed at 1.5 mm tail diameter to induce moderate bleeding. Tails were immersed in 50 mL of saline at 37 degrees. Total blood loss was measured as the blood volume collected during 20 min normalized for mouse weight (microL/g). Mice given only anti-FVIII antibody had more blood loss (median = 6.7 microL/g) compared to control mice (median < 2 microL/g) (Figure). In this mouse model receiving anti-FVIII antibody, CM (5.4 mg/kg) injected at 15 min prior to tail cutting significantly reduced the median blood loss from 6.7 to 2.0 and 3.2 microL/g, respectively (p < 0.001 for each myosin) (Figure). Thus, these studies provide in vivo proof of concept that both CM and SkM can reduce bleeding and are procoagulant in vivo.
Second, studies of the effects of CM on thrombogenesis ex vivo using fresh human flowing blood showed that perfusion of blood over CM-coated surfaces at 300 s-1 shear rate caused extensive fibrin deposition. Addition of CM to blood also promoted the thrombotic responses of human blood flowing over collagen-coated surfaces, evidence of CM's thrombogenicity. Further studies showed that CM enhanced thrombin generation in platelet rich plasma and platelet poor plasma, indicating that CM promotes thrombin generation in plasma primarily independently of platelets.
To address the mechanistic insights for CM's procoagulant activity, purified coagulation factors were employed. In a purified system composed of factor Xa, factor Va, prothrombin and calcium ions, CM greatly enhanced prothrombinase activity. Experiments using Gla-domainless factor Xa showed that the Gla domain of factor Xa was not required for CM's prothrombinase enhancement in contrast to phospholipid-enhanced prothrombinase activity which requires that Gla domain. Binding studies showed that CM directly binds factor Xa. In summary, here we show that CM is procoagulant due to its ability to bind factor Xa and strongly promote thrombin generation.
In summary, CM acts as procoagulant by its ability to bind factor Xa and strongly promote thrombin generation both in vivo an in vitro. These provocative findings raise many questions about whether and how the protective pro-hemostatic properties or the pathogenic prothrombotic properties of CM contribute to pathophysiology in the coronary circulation. This discovery raises many questions about CM and coronary pathophysiology, and future CM research may enable novel translations of new knowledge regarding CM's procoagulant activities for coronary health and disease.
Mosnier:The Scripps Research Institute: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Ruggeri:MERU-VasImmune Inc.: Equity Ownership, Other: CEO and Founder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal