Introduction: Nephropathy, one of the most common complications of sickle cell disease (SCD), shortens life expectancy and accounts for 16-18% of SCD-associated deaths. SCD nephropathy is caused by recurrent vaso-occlusions (Am. J. Hematol. 2014;89:907) and by hemolytic anemia, which result in chronic tissue hypoxia, inflammation, and ultimately organ damage (Haematologica. 2012;97(2):201; Pediatr. Nephrol. 2014;29(10):1997). Reduced bioavailability of the gasotransmitter nitric oxide (NO) is one of the consequences of red blood cell hemolysis in SCD. NO insufficiency dysregulates the NO-soluble guanylyl cyclase (sGC)- cyclic guanosine monophosphate (cGMP) signaling pathway, promoting vasoconstriction, inflammation, and assembly of hetero cellular aggregates on the vascular wall, which occlude venous microcirculation. Olinciguat, a stimulator of soluble guanylate cyclase, increases NO-induced synthesis of the second messenger cGMP. In preclinical models, olinciguat has been shown to induce vasorelaxation and elicit anti-inflammatory and reno-protective effects. In an SCD mouse model of vaso-occlusive crisis (VOC), olinciguat reduced leukocyte/endothelial cell interactions, improved blood flow, and increased survival time (EHA 2019, PS1521). Here we evaluated the effect of chronic olinciguat treatment on biomarkers of inflammation and renal injury in an SCD mouse model.
Methods: 18-week-old Townes non-disease control (HbAA) and SCD mice (HbSS) were studied. SCD mice were twice daily orally administered with either vehicle or olinciguat (20 mg/kg/day) for 8 weeks. Animals were placed in metabolic cages at 8 weeks for 24-hour urine collection. At the end of the study, plasma and kidneys were collected for biomarker analysis.
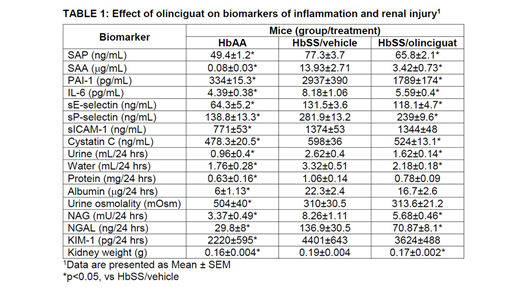
Results: Plasma concentrations of the inflammatory biomarkers serum amyloid P component (SAP), serum amyloid A (SAA), interleukin 6 (IL-6), and plasminogen activator inhibitor 1 (PAI-1) were higher in SCD mice compared to non-disease control mice. SCD mice treated with olinciguat had significantly (p<0.05) lower levels of SAP, SAA, IL-6, and PAI-1 as compared to SCD control mice. Plasma levels of soluble adhesion receptors were also higher in SCD mice than non-disease control mice which indicate endothelial activation. SCD mice that were treated with olinciguat had significantly lower levels of plasma sE-selectin and sP-selectin compared to SCD control mice. There was no effect of treatment on sICAM-1. Cystatin C, a plasma biomarker of renal function, was significantly higher in SCD mice as compared to non-disease control mice; while mice treated with olinciguat had significantly lower levels compared to SCD control mice. Compared to non-disease control mice, SCD mice had proteinuria and albuminuria along with increased urine output and water consumption. Urine protein and urine albumin were lower in mice treated with olinciguat yet these data did not reach statistical significance (p=0.053 and p=0.065, respectively). Urine excretion was significantly lower in SCD mice treated with olinciguat as compared to SCD control mice. Olinciguat-treated mice also consumed significantly less water than mice from the SCD control mice. As compared to healthy control mice, SCD mice had significantly lower urine osmolality indicating tubular dysfunction while olinciguat treatment had no effect on urine osmolality. Analysis of biomarkers of kidney injury demonstrated that, relative to healthy control mice, urinary excretion of NAG, NGAL and KIM-1 was significantly higher in SCD mice. SCD mice treated with olinciguat had significantly less urinary excretion of NAG and NGAL as compared to SCD control mice. KIM-1 was not affected by olinciguat treatment. Kidney weight was significantly increased in sickle cell mice as compared to healthy control mice. Olinciguat-treated SCD mice had significantly lower kidney weight than SCD mice from vehicle control group.
Conclusions: In summary, treatment of SCD mice with the sGC stimulator olinciguat elicited lower levels of plasma biomarkers of inflammation and endothelial cell activation and biomarkers of kidney injury.
Tchernychev:Cyclerion Therapeutics: Employment, Equity Ownership. Liu:Cyclerion Therapeutics: Employment, Equity Ownership. Hall:Cyclerion Therapeutics: Employment, Equity Ownership. Graul:Cyclerion Therapeutics: Employment, Equity Ownership. Masferrer:Cyclerion Therapeutics: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal