Introduction:
We used MR imaging techniques to investigate the neurological consequences of chronic anemia in human patients with sickle cell disease, non-sickle anemic syndromes (called anemic-controls) and non-anemic controls. We previously demonstrated that hemoglobin level is an independent predictor of white matter volume (WMV) in brain as well as cognitive performance. Importantly, WMV was independent of genotype (sickle vs non-sickle), treatment type, HgB S%, fetal HgB level, Lactate dehydrogenase (LDH) and presence of silent strokes. We also demonstrated that low hemoglobin is associated with abnormal brain functional connectivity and iron levels in select brain regions.
In the present study, we examine the effects of anemia on brain integrity using apparent diffusion coefficient (ADC) calculated from diffusion weighted imaging (DWI). ADC measures the magnitude of the motion of water in tissue and can be used to imply tissue damage, making it highly sensitive to neuropathology as altered tissue integrity and loss of cellular structures can change the diffusivity of water. We then explore the associations between ADC, local brain volume, functional connectivity, iron and cognitive performance.
Methods:
MRI data, CBC and neuropsychological testing results were obtained from 26 sickle cell disease (age = 20.9 ± 11.3, F:M = 13:13, HgB = 9.7 ± 2.1), 20 anemic-control (age = 25.9 ± 11.3, F:M = 10:10, HgB = 10.9 ± 0.5) and 25 control subjects (age = 23.1 ± 8.4, F:M = 16:9, HgB = 13.2 ± 1.2). (Recruited with informed consent or assent; IRB: CHLA CCI#11-00083).
DWI (TE = 86ms; TR = 6700ms; resolution=2.5mm3; 30 directions; bvalue=1000m/s2; reverse-gradient b=0) were acquired on a 3T Philips Achieva (v.3.2.1) using an 8-channel head coil. Images were corrected for motion and distortion then voxel-wise calculations of ADC were computed then transferred to a common atlas space. (brainsuite.org, v18). 3D T1 weighted, quantitative susceptibility mapping, and functional MRI (fMRI) images were collected using our previously published protocols.
After regressing out age and sex, we determined the effect of hemoglobin level at each voxel of the brain on ADC then corrected for multiple comparison (BH FDR α=0.1). Significant regions were retained, (p<0.05), and a mean ADC was computed for each subject from those regions. Pearson's correlations were used to determine the effects of hemoglobin level on the following measures: (1) mean ADC, (2) WMV, (3) Connectivity Dissimilarity Index (CDI), a measure quantifying the dissimilarity of functional connectivity pattern between each subject and a reference fMRI atlas (4) Iron measured in the substantia nigra of the brain's basal ganglia through R2* and susceptibility images and (5) Matrix Reasoning, a nonverbal measure of novel problems (fluid reasoning).
Results:
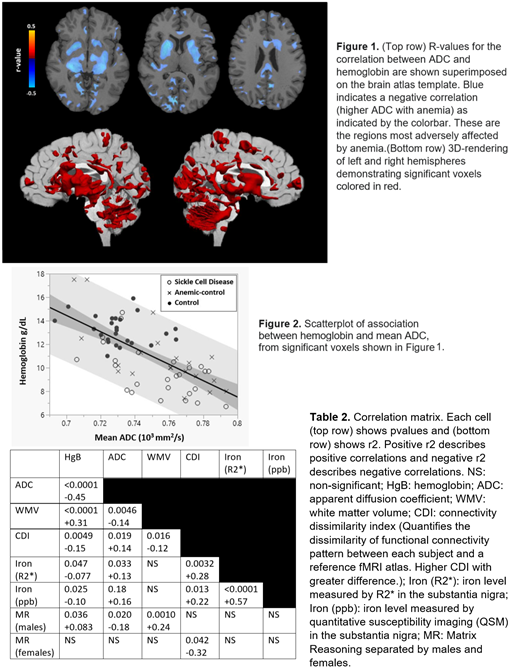
Hemoglobin level significantly correlated with ADC throughout the brain, but most strongly in deep white matter and subcortex, followed by the occipital lobe and cerebellum (Figure 1). The scatterplot between mean ADC and hemoglobin showed no discernable differentiation between anemia subtypes (Figure 2). Mean ADC and our previously developed markers of disease correlated well with each other showing that the severity of anemia correlates with higher ADC, lower white matter volume (WMV), abnormal functional connectivity (CDI), higher brain iron and lower Matrix Reasoning scores (males only; Table 1).
Conclusion:
Increased ADC, correlating with anemia severity, was observed in subcortical structures of an anemic population at risk for white matter shrinkage and cognitive dysfunction. ADC (but not WMV) correlated with brain iron which is known to accumulate in the presence of cerebral hypoxia. ADC and WMV changes were comparable in males and females but only males showed lower fluid reasoning. This data shows that anemia and brain iron are associated with brain tissue disruption and function.
Coates:vifor: Consultancy, Honoraria; celgene: Consultancy, Honoraria, Other: steering committee of clinical study; agios pharma: Consultancy, Honoraria; apo pharma: Consultancy, Honoraria, Speakers Bureau. Wood:BiomedInformatics: Consultancy; Imago Biosciences: Consultancy; National Institutes of Health: Research Funding; Apopharma: Consultancy; WorldcareClinical: Consultancy; Philips Healthcare: Research Funding; BluebirdBio: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal