Background. A preliminary study involving 59 patients demonstrated that pancreatic iron assessed by magnetic resonance imaging (MRI) was the strongest overall predictor of glucose dysregulation in thalassemia major (TM) patients.
Aim. In the present multicenter study we explored systematically the link between pancreatic iron and glucidic metabolism in a large cohort of TM patients.
Methods. We considered 705 TM patients (372 F, mean age 37.00±9.95 years) enrolled in the E-MIOT (Extension-Myocardial Iron Overload in Thalassemia) project. T2* measurements were performed over pancreatic head, body and tail and global value was the mean. The pattern of disturbances of glucose metabolism was assessed by means of the oral glucose tolerance test (OGTT).
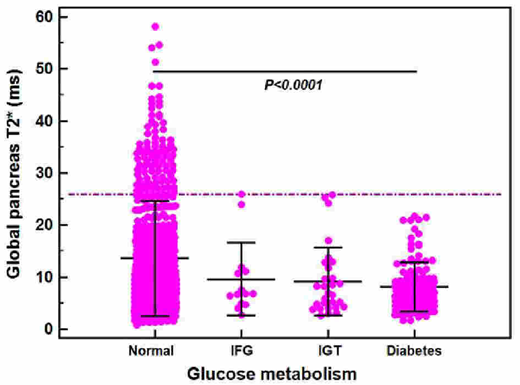
Results. According to OGTT results, 546 patients (77.4%) had normal glucose tolerance (NGT), 14 (2.0%) had isolated impaired fasting glucose (IFG), 29 (4.1%) had impaired glucose tolerance (IGT), and 116 (16.5%) had diabetes mellitus (DM).
None of the 85 patients (12.1%) without pancreatic iron overload (global pancreas T2*≥26 ms) had IGT or DM (Figure 1). The 84.6% of patients with NGT had pancreatic iron overload. The global pancreatic T2* values were significantly higher in patients with NGT than in patients with DM (13.58±11.08 ms versus 8.09±4.72 ms, P<0.0001).
Receiver operator characteristic (ROC) analysis showed that a global pancreas T2*<13.73 ms was the optimal cutoff for predicting an abnormal OGTT, with an area under the curve (AUC) of 0.62.
Global pancreas T2* values showed a weak significant correlation with insulin values (R=0.160; P=0.002) and homeostasis model assessment-insulin resistance (HOMA-IR) index (R=0.122; P=0.019).
Conclusion. A normal global pancreas T2* value has a negative predictive value of 100% for IGT and DM. The low specificity of pancreatic iron overload for glucose dysregulation seems to support the hypothesis that a latency time is need before pancreatic iron burden could give impaired glucose tolerance and overt diabetes mellitus.
Pepe:Chiesi Farmaceutici S.p.A., ApoPharma Inc., and Bayer: Other: No profit support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal