Patients with transfusion-dependent β-thalassaemia (TDT) require regular red blood cell (RBC) transfusions to treat anaemia, with regular monitoring and obligatory iron chelation therapy to address inevitable iron overload.
We conducted a retrospective chart review with cross-sectional patient and caregiver surveys in 9 National Health Service (NHS) centres across the United Kingdom (UK) to evaluate routine management of TDT, and patient and caregiver health-related quality of life (HRQoL). Eligible patients had a documented diagnosis of TDT (index event) ≥2 years prior to data collection. The observation period was the 2-5 year period prior to data collection or death. Patient-reported outcomes (PRO) were completed at enrolment, including EuroQoL EQ-5D, Work Productivity and Activity Impairment (WPAI), and disease-specific TranQoL. The primary endpoint was the number of blood transfusion episodes per patient per year.
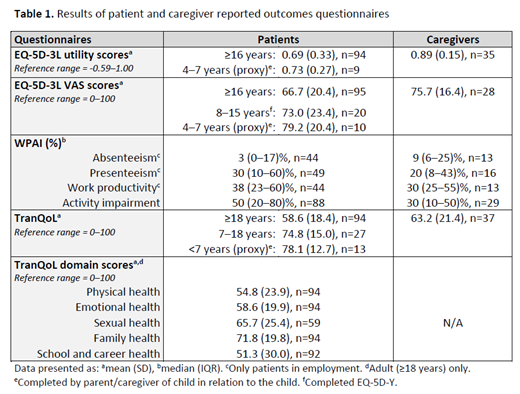
A total of 165 patients were included (median age at data collection 24.1 [interquartile range (IQR) 11.8-37.2] years; 50% (n=82) male; one patient was deceased); 135 patients completed ≥1 PRO questionnaire; 37 caregivers completed caregiver questionnaires. Median age at index event was 1.0 (IQR 0.5-8.0) years and median disease duration was 11.6 (IQR 6.4-21.7) years. The most commonly recorded comorbidities (n=156 patients, at start of observation period) included hypogonadotrophic hypogonadism (20% [n=31]), vitamin D deficiency (16% [n=25]) and osteoporosis (14% [n=22]). Twenty percent (n=31) were splenectomised. During a mean observation period of 4.7 (standard deviation [SD] 0.7) years, patients had a mean of 13.5 (SD 3.6) transfusion episodes per year (79% had ≥12 per year) and a median of 32.4 (IQR 25.3-37.7) units transfused per year. Mean pre-transfusion haemoglobin (Hb, from 8041 tests) was 99.1 (SD 10.0) g/L; patients had a mean of 10.3 (SD 5.0) Hb tests per year. Median age at initiation of chelation therapy (n=91) was 2.9 (IQR 1.8-12.1) years. All patients received ≥1 iron chelator during the observation period, including deferasirox (80% [n=132]), desferrioxamine (52% [n=85]), deferiprone (35% [n=57]) or combination therapies (20% [n=33]). At data collection 161 patients were receiving chelators. Patients had a mean of 10.7 (SD 4.3) serum ferritin (SF) measurements/year; median SF closest to data collection was 1792.0 (IQR 997.0-2883.0) ng/ml (≤1000 ng/ml [25%, n=42]; >1000≤2500 ng/ml [39%, n=64]; >2500 ng/ml [36%, n=59]). Median interval between liver iron concentration (LIC) tests was 1.9 (IQR 1.3-2.5) years; median R2 LIC closest to data collection (n=120) was 5.4 (IQR 2.9-11.5) mg/g (<7 mg/g [61%, n=73]; 7<15 mg/g [23%, n=28]; ≥15 mg/g [16%, n=19]). Median interval between cardiac MRI was 2.5 (IQR 1.7-5.0) years; median T2* cardiac iron closest to data collection (n=132) was 30.3 (IQR 22.0-37.1) ms (>20 ms [80%, n=105]; 10-20 ms [11%, n=14]; <10 ms [10%, n=13]).. PRO and caregiver questionnaire results are shown in Table 1. Mean EQ-5D utility score for adult patients (≥16 years, n=94) was 0.69 (SD 0.33; individual domain scores: mobility [n=97] 58% no problems, 42% some problems; self-care [n=98] 79% no problems, 21% some problems; usual activities [n=95] 48% no problems, 42% some problems, 9% unable to perform; pain/discomfort [n=98] 43% none, 46% moderate, 11% extreme; and anxiety [n=98] 62% none, 33% moderate, 5% extreme). Median (IQR) WPAI domain scores for adult patients (≥18 years) included work productivity loss (n=44) of 38% (23-60%) and activity impairment (n=88) of 50% (20-80%); caregivers reported median (IQR) work productivity loss (n=13) of 30% (25-55%) and activity impairment (n=29) of 30% (10-50%). Mean TranQoL score for adult patients (≥18 years, n=94) was 58.6 (SD 18.4).
These results offer insights into the real-world management of patients with TDT in the UK NHS, and the burden placed on patients, caregivers and healthcare resources. In general patients appear to have been well managed; however, a subset of patients suffer severe liver- and cardiac-iron loading, the latter being associated with significant mortality risk. All patients received chelation therapy, and 20% of patients required combination chelation therapy. The impact of the disease on the patient/caregiver should not be underestimated as evidenced by the PRO data.
Shah:Abfero pharmaceuticals: Other: clinical safety committee; Celgene: Other: clinical trial steering committee; Silence therapeutics: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Telfer:ApoPharma: Membership on an entity's Board of Directors or advisory committees, Other: Speaker activities, clinical trial activities; Pfizer: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: clinical trial activity; Terumo: Honoraria, Other: Speaker activity; Celgene: Other: clinical trial involvement; Kyowa Kirin Limited: Research Funding; Bluebird Bio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Napp Pharma: Other: clinical trial involvement; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: clinical trial activities. Velangi:bluebird bio: Membership on an entity's Board of Directors or advisory committees. Pancham:Celgene: Membership on an entity's Board of Directors or advisory committees, Other: sponsorship to attend meeting. Pollard:bluebird bio: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: support to attend educational meetings. Chalmers:Novartis: Consultancy. Kell:Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Hickey:OPEN VIE: Employment. Paramore:bluebird bio: Employment, Other: owns stock in the company. Jobanputra:bluebird bio: Employment, Other: owns stock in the company. Ryan:Novartis: Other: educational grant; Pfizer: Membership on an entity's Board of Directors or advisory committees; bluebird bio: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal