Introduction
Eculizumab (Ecu) is the standard treatment for paroxysmal nocturnal hemoglobinuria (PNH), as it results in sustained control of intravascular hemolysis and prevention of thrombosis, and significant improvement of long-term survival. However, the hematological benefits remain heterogeneous among patients, and most response analyses are restricted to transfusion independence. In the era of new anti-complement therapies, we found appropriate to investigate in detail the quality of hematological response of Ecu-treated PNH patients.
Methods
We have recently proposed (Risitano et al, Frontiers in Immunology 2019) a classification of hematological response to anti-complement therapies based on hemoglobin (Hb) levels and laboratory markers of hemolysis. Six distinct categories were determined (Table 1): i. complete response (no transfusion requirement with normal stable Hb and no evidence of hemolysis); ii. major response (no transfusion requirement with normal Hb but evidence of residual intravascular or extravascular hemolysis); iii. good response (no transfusion requirement with persistent chronic mild anemia); iv. partial response (persistent chronic moderate anemia and/or with occasional red blood cell transfusions); v. minor response (3-6 red blood cell transfusions every 6 months); vi. no response (>6 blood cell transfusions every 6 months). Here we have retrospectively exploited this classification to assess hematological response in a large cohort of 93 PNH patients evaluated in six international reference PNH centers (Paris, Naples, London, Florence, São Paulo, and Ribeirão Preto).
Results
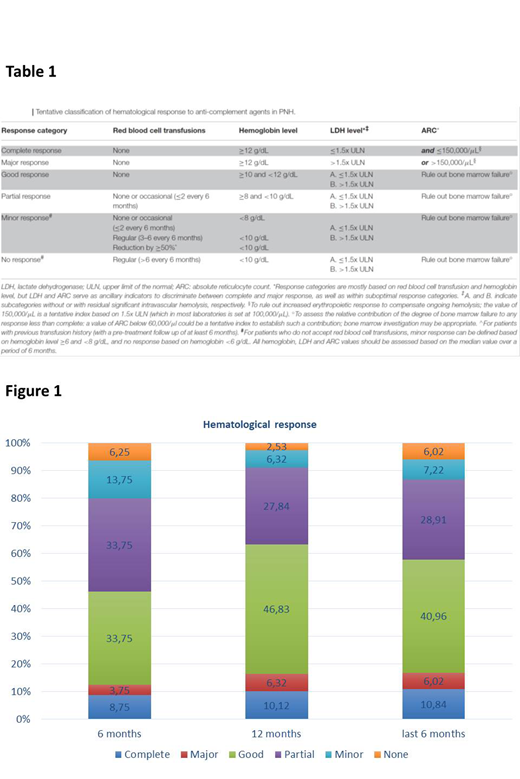
Forty-two patients were male and 51 were female; the median age at diagnosis (treatment starting) was 36 years (range, 12 to 74 years). All the patients have started Ecu treatment for hemolytic PNH; 57% of patients had classic PNH, whereas 30% and 13% had aplastic anemia (AA)/PNH or intermediate PNH (Peffault de Latour et al, Blood 2006), respectively. Two-thirds of patients had been receiving red blood cell (RBC) transfusion before starting Ecu; the mean treatment duration was 97 months (range 6-179). The majority of patients (87%) received Ecu at the standard schedule of 900 mg every 2 weeks; due to chronic, residual, intravascular hemolysis 13% were treated with higher doses (1200 mg) or with shorter administration interval (10-12 days). Hematological responses were evaluated early during the treatment course (at 6 and 12 months), and then later during the last 6 months of treatment (Figure 1). After 6 (n=80) and 12 months (n=79) of Ecu, respectively, rates of hematological response were as follows: complete response 9% and 10%, major response 4% and 6%, good response 34% and 47%, partial response 34% and 28%, minor response 14% and 6% and no response 6% and 3%. In 83 patients treated for more than 18 months late response rates also were evaluated; most patients responded and the rates of hematological response during the last 6 months of treatment were as follows: complete response, 11%; major response, 6%; good response, 41%; partial response, 29%; minor response, 7% and no response, 6%. Breakthrough episodes were recorded in 21% of patients (equally split between pharmacokinetic and pharmacodynamic ones); thromboembolic events and AA were seen in 2% and 3% of patients, respectively. These data suggest that, albeit individual and unpredictable, the extent of the hematological benefit of Ecu in most PNH patient can be determined quite early in the treatment course, after a minimum of 12 months.
Conclusion
This is the first attempt to classify hematological response to Ecu based on Hb levels and residual hemolysis. The proposed classification allows the identification of distinct patient subgroups with different medical needs, as well as of meaningful clinical goals for future anti-complement therapies. In our cohort, about 60% of patients are well controlled with Ecu, even if less than 20% of patients reach normal Hb values; about 40% of patients remain anemic, and this represents a goal for future therapies in PNH. This classification is helpful to characterize more precisely the clinical response, which should be assessed after 1 year of treatment. Even if it should be validated in independent, larger cohorts, this classification represents a useful tool to design future trials, and to compare the clinical results of the novel anti-complement agents in development for PNH.
Notaro:Alexion: Membership on an entity's Board of Directors or advisory committees, Other: letture fees. Scheinberg:Pfizer,: Speakers Bureau; Celgene: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kulasekararaj:Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Achilleon: Consultancy; Ra Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Akari Therapeutics: Consultancy. Risitano:Biocryst: Membership on an entity's Board of Directors or advisory committees; Samsung: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Ra Pharma: Research Funding; Samsung: Membership on an entity's Board of Directors or advisory committees; Achillion: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ra Pharma: Research Funding; Alnylam: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alnylam: Research Funding; Amyndas: Consultancy; Achillion: Research Funding; Amyndas: Consultancy; Biocryst: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees. Peffault de Latour:Pfizer: Consultancy, Honoraria, Research Funding; Alexion: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal