Background: PNH is a rare, acquired blood disorder. A somatic mutation in the PIGA gene of one or more hematopoietic stem cells generates a clone of abnormal erythrocytes (RBCs) that lack alternative pathway (AP) regulatory complement proteins CD55 and CD59. This leads to uncontrolled complement activation on affected RBCs and intravascular hemolysis (IVH).
Standard of care is C5 inhibition to prevent membrane attack complex (MAC) formation. Despite preventing MAC-mediated IVH, many patients experience extravascular hemolysis (EVH). Despite C5 inhibition, ~ 70% of patients remain anemic and >1/3 were transfused > 1 time in the prior 12 months.
Factor D (FD), a serine protease, catalyzes complement factor B cleavage, allowing formation of AP C3 convertase. By inhibiting FD, oral danicopan blocks C3 convertase formation, the control point for AP activation and amplification of all pathways. This leads to inhibition of C3 cleavage, C3 fragment deposition, terminal pathway activation and MAC formation. Thus, danicopan can control both IVH and EVH therefore, making FD a promising target.
Aim: Demonstrate that danicopan is a potential treatment for PNH patients with an inadequate response to C5 inhibition.
Methods: Data are presented for this Ph 2, dose-finding, proof of concept trial of danicopan in patients with an inadequate response to eculizumab who were transfusion dependent. Additional criteria in Table 1.
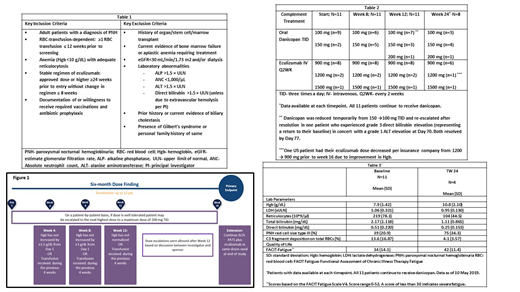
In addition to the current eculizumab regimen, danicopan starting doses were 100-150 mg TID, with dose escalation up to 200 mg TID, based on clinical and biochemical response, at protocol defined time points (Figure 1).
The primary endpoint was change in Hgb at Treatment Week (TW) 24. Secondary efficacy parameters included transfusion needs, effect on lactate dehydrogenase (LDH), and an exploratory an endpoint of Functional Assessment of Chronic Illness Therapy (FACIT) FATIGUE scores. General safety, tolerability, and PK/PD of danicopan were measured. After TW 24, patients entered a long-term extension.
Results: Twelve patients received at least one dose of danicopan. One patient discontinued after 2 doses, due to a serious adverse event of worsening of an underlying condition (pulmonary hypertension/edema), considered unlikely related to danicopan. This patient is excluded from this analysis. Eleven patients continue to receive treatment. Dose titrations are shown in Table 2.
Benefits were observed in multiple laboratory markers of PNH, shown in Table 3. Hgb improved in all patients, with a mean Hgb gain of 2.6 g/dL at 24 Weeks of treatment (n=4). Meaningful improvement in FACIT Fatigue scores were reported, with a mean increase of 8 points relative to the baseline on eculizumab. A 3-point change is clinically meaningful on this scale.
Transfusion needs dramatically reduced, with one patient receiving one transfusion during the trial, as compared to 34 transfusions (58 units) in 10 patients, in the 6 months prior to screening.
One patient (who does not accept blood products due to religious objections and also has hereditary elliptocytosis) had a baseline Hgb=5 g/dL with a 3.5 g/dL improvement at TW24 and significant improvement in fatigue.
C3 fragment deposition was inhibited; reticulocytes, total/ direct bilirubin and LDH returned to normal range. Baseline C5 inhibition (classical pathway activity) was essentially inhibited (mean=1 [range 60-144]). Three of 11 patients received > 900 mg eculizumab, making PK a less likely reason for inadequate response.
Danicopan has been well tolerated; 96% of treatment emergent adverse events (TEAEs) were mild to moderate in severity and no discontinuations due to TEAEs. Two patients had Grade 3 TEAEs which resolved and continued in the trial.
Conclusion: Proof of concept is established with danicopan, an oral, small molecule FD inhibitor in the treatment of PNH in combination with eculizumab. Meaningful improvements in Hgb, transfusion needs, FACIT-FATIGUE and other parameters of interest were achieved. These clinically significant improvements demonstrate that further benefit can be achieved by blocking the alternative pathway at FD with danicopan, in combination with C5 inhibition. This benefit is likely due to the prevention of C3-mediated EVH, in addition to control of IVH. Danicopan targets an unmet need in PNH and will be further evaluated in a pivotal trial in combination with standard of care C5 inhibition.
Kulasekararaj:Achillion: Consultancy; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Ra Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Akari: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alnylam: Membership on an entity's Board of Directors or advisory committees. Risitano:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amyndas: Consultancy; Achillion: Research Funding; Amyndas: Consultancy; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Samsung: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Research Funding, Speakers Bureau; Alexion: Honoraria, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Research Funding; Achillion: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ra Pharma: Research Funding; Biocryst: Membership on an entity's Board of Directors or advisory committees; Alnylam: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Biocryst: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ra Pharma: Research Funding; Samsung: Membership on an entity's Board of Directors or advisory committees; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Maciejewski:Novartis: Consultancy; Alexion: Consultancy. Notaro:Alexion: Membership on an entity's Board of Directors or advisory committees, Other: letture fees. Browett:Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Beigene: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Achillion: Research Funding. Lee:Alexion: Consultancy, Honoraria, Research Funding; Achillion: Research Funding. Huang:Achillion: Employment, Equity Ownership. Geffner:Achillion: Employment, Equity Ownership. Brodsky:Alexion: Membership on an entity's Board of Directors or advisory committees, Other: Grant funding; Achillion: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal