Background: Pyruvate kinase (PK) deficiency is a congenital hemolytic anemia caused by mutations in the PKLR gene, leading to a deficiency of the glycolytic enzyme red cell PK (PK-R). Current treatments for PK deficiency are supportive only. Mitapivat (AG-348) is an oral, small-molecule, allosteric PK-R activator in clinical trials for PK deficiency. We previously described results from DRIVE PK, a phase 2, randomized, open-label, dose-ranging study in adults with PK deficiency (N=52) treated with mitapivat for a median of 6 months.
Aim: To report long-term safety and efficacy of mitapivat in patients who continue treatment in the ongoing Extension period of the DRIVE PK study (ClinicalTrials.gov NCT02476916).
Methods: Patients were eligible to participate if ≥18 years of age with a confirmed diagnosis of PK deficiency (enzyme and molecular testing); baseline hemoglobin (Hb) levels ≤12.0 g/dL (males) or ≤11.0 g/dL (females); and if they had not received more than 3 units of red blood cells in the prior 12 months, with no transfusions in the prior 4 months. Patients were initially randomized 1:1 to receive mitapivat 50 mg twice daily (BID) or 300 mg BID for a 6-month Core period. Dose adjustment was allowed during the Core period based on safety and efficacy. Patients experiencing clinical benefit without concerning safety issues related to mitapivat (investigator discretion) could opt to enter the Extension period, with follow-up visits every 3 months. Safety (adverse events [AEs]) and efficacy (hematologic parameters including Hb) were assessed. Protocol amendments during the Extension period required that (1) patients who did not have an increase from baseline Hb of ≥1.0 g/dL for ≥3 of the prior 4 measurements withdraw from the study, and (2) patients treated with mitapivat doses >25 mg BID undergo a dose taper and continue on the dose that maintained their Hb level no lower than 1.0 g/dL below their pre-taper Hb level.
Results: Fifty-two patients enrolled in this study and were treated in the 24-week Core period; 43 (83%) patients completed the Core period and 36 (69%) entered the Extension period. Eighteen patients discontinued from the Extension period: investigator decision (n=8), AEs (n=1), consent withdrawal (n=1), noncompliance (n=1), or other (n=7). Thus, 18 patients, all of whom received ≥29 months of treatment with mitapivat (median 35.6, range 28.7-41.9) have continued treatment. Ten of these 18 patients were male, 11 had a prior splenectomy, and 5 had a history of iron chelation. Median age was 33.5 (range 19-61) years; mean baseline Hb was 9.7 (range 7.9-12.0) g/dL. All patients had ≥1 missense PKLR mutation. The doses (post-taper) at which treatment was continued were (BID): ≤25 mg (n=12), 50 mg (n=5), and 200 mg (n=1).
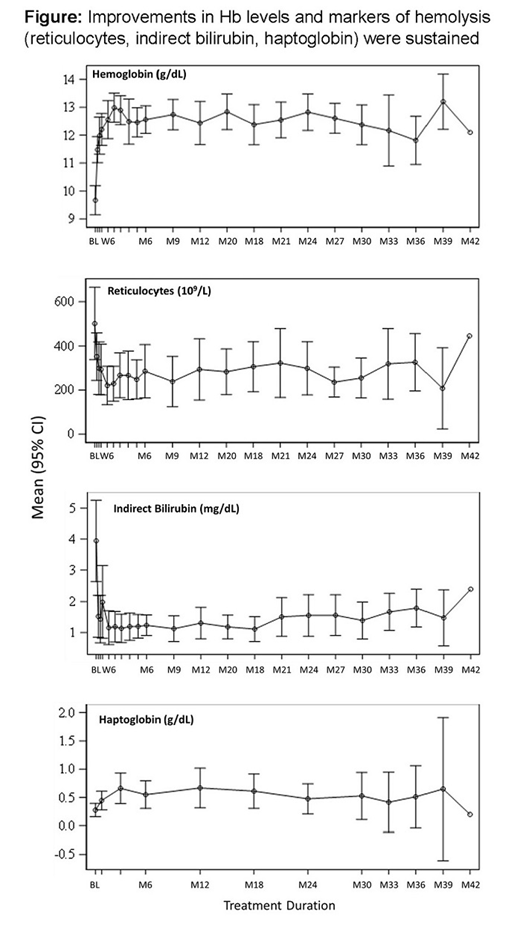
Improvements in Hb levels and markers of hemolysis (reticulocytes, indirect bilirubin, haptoglobin) were sustained (Figure). Among the 18 patients, headache was the most commonly reported AE during both the Extension (n=7, 38.9%) and Core (n=10, 55.6%) periods. Reports of insomnia and fatigue during the Extension period (n=5, 27.8% each) were the same as or similar to those during the Core period. There were fewer reports of nausea (2 vs 6) and hot flush (0 vs 5) in the Extension period. Nasopharyngitis was reported in 5 patients in the Extension period vs 1 patient in the Core period. These data are consistent with the AE profile for the 52 patients treated overall in the Core period, in that headache (44%), insomnia (40%), and nausea (38%) were the most commonly reported AEs and were transient (generally resolved within 7 days without intervention).
Conclusion: Chronic daily dosing with mitapivat for a median of 3 years was well tolerated, with no new safety signals reported. Increased Hb levels and improvements in hemolysis markers were sustained at the optimized individual doses. These long-term data support the potential of mitapivat as the first disease-altering therapy for PK deficiency. Two phase 3 trials are underway to further study the effect of mitapivat in patients with PK deficiency.
Grace:Novartis: Research Funding; Agios Pharmaceuticals, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Layton:Novartis: Membership on an entity's Board of Directors or advisory committees; Cerus Corporation: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees. Galactéros:Addmedica: Membership on an entity's Board of Directors or advisory committees. Barcellini:Novartis: Research Funding, Speakers Bureau; Alexion: Consultancy, Research Funding, Speakers Bureau; Apellis: Consultancy; Incyte: Consultancy, Other: Advisory board; Agios: Consultancy, Other: Advisory board; Bioverativ: Consultancy, Other: Advisory board. van Beers:Agios Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; RR Mechatronics: Research Funding. Ravindranath:Agios Pharmaceuticals, Inc.: Other: I am site PI on several Agios-sponsored studies, Research Funding. Kuo:Agios: Consultancy; Alexion: Consultancy, Honoraria; Apellis: Consultancy; Bioverativ: Other: Data Safety Monitoring Board; Bluebird Bio: Consultancy; Celgene: Consultancy; Novartis: Consultancy, Honoraria; Pfizer: Consultancy. Sheth:Apopharma: Other: Clinical trial DSMB; CRSPR/Vertex: Other: Clinical Trial Steering committee; Celgene: Consultancy. Kwiatkowski:bluebird bio, Inc.: Consultancy, Research Funding; Apopharma: Research Funding; Novartis: Research Funding; Terumo: Research Funding; Celgene: Consultancy; Imara: Consultancy; Agios: Consultancy. Hua:Agios Pharmaceuticals, Inc.: Employment, Equity Ownership. Hawkins:Bristol Myers Squibb: Equity Ownership; Infinity Pharma: Equity Ownership; Agios: Employment, Equity Ownership; Jazz Pharmaceuticals: Equity Ownership. Mix:Agios: Employment, Equity Ownership. Glader:Agios Pharmaceuticals, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal