Background: Hypomethylating agents (HMA), azacitidine (AZA) and decitabine (DEC), are the current standard of care in patients with intermediate-2 and high risk myelodysplastic syndromes (MDS) by IPSS prognostic category (higher-risk MDS) based on clinical trials showing increased response rates and overall survival with these agents. However, the outcomes of patients with MDS treated with HMAs have not been comprehensively assessed in real-world practice. This study describes real-world treatment patterns and outcomes among patients with MDS treated with HMAs.
Methods: Adult patients with a confirmed MDS diagnosis between 01/2009-12/2015 treated with HMA were identified in the SEER-Medicare database (01/2006-12/2016). The index date was the date of HMA initiation; the first HMA was defined as the index HMA. Patients were required to have Medicare Parts A and B coverage for ≥12 months pre-index and ≥1 month post-index date. Patients with evidence of stem cell transplant (SCT) or acute myeloid leukemia (AML) pre-index, or enrollment in a clinical trial at any time were excluded. Patients meeting the aforementioned criteria formed the study population.
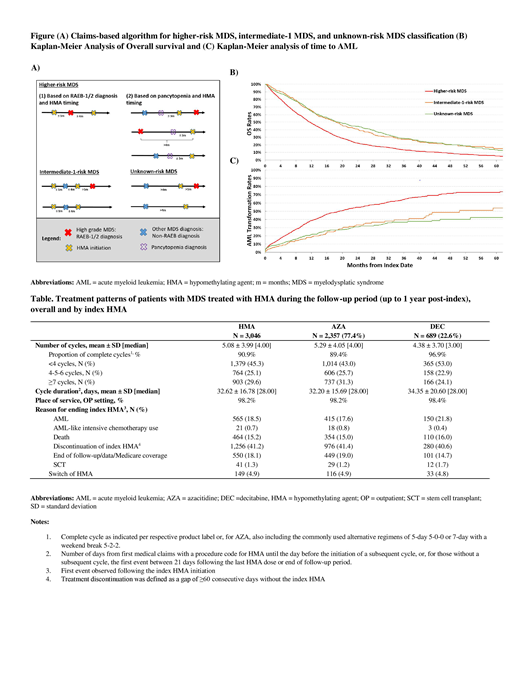
An algorithm, based on timing of HMA initiation, timing of diagnosis of pancytopenia (low counts for red blood cells, white blood cells, and platelets), and diagnosis of refractory anemia with excess of blasts (RAEB)-1 and RAEB-2, was developed to categorize patients into specific MDS risk groups using claims data (Figure A). Patients with RAEB-1/2 diagnosis who initiated HMA within 1 month prior to or 4 months post-RAEB diagnosis, or pancytopenia diagnosis within 3 months prior to HMA initiation (regardless of types of MDS diagnosis) were classified into the higher-risk MDS group. Patients with non-RAEB MDS diagnosis and HMA initiation within 1 month prior to or 4 months post-MDS diagnosis were classified into the intermediate-1-risk MDS group; remaining, unclassified patients from the study population were assigned into the unknown-risk MDS group. OS and time to AML transformation (determined by a diagnosis of AML in claims) were assessed for each risk stratum post-index using Kaplan-Meier (KM) analyses. HMA treatment patterns were measured up to 12 months post-index or until the first event among the following: SCT, AML-like intensive chemotherapy use, AML, or end of data/Medicare Parts A and B coverage (follow-up period).
Results: A total of 3,046 patients with MDS treated with HMA were included. On average, patients were aged 77.4 years, and 36.8% were female. The majority of patients were classified in the higher-risk MDS group (70.9%), 8.0% in the intermediate-1-risk MDS group, and 21.1% in the unknown-risk MDS group. Median OS was 11.6 months among patients in the higher-risk MDS group, whereas median OS was 18.4 and 19.1 months for patients in the intermediate-1 and unknown-risk MDS groups, respectively (Figure B). Median time to AML transformation was 19.3 months in the higher-risk MDS group, 50.4 months in the intermediate-1-risk MDS group, and was not reached for the unknown-risk MDS group (Figure C).
Overall, patients received an average of 5.1 HMA cycles (median = 4.0 cycles) and the majority were complete cycles (90.9%; as indicated per label or, for AZA, also including the commonly used alternative regimens of 5-day 5-0-0 or 7-day with a weekend break 5-2-2). The mean duration of HMA cycles was 32.6 days (median = 28.0 days). As many as 45.3% of patients received <4 cycles, 41.2% eventually discontinued the index HMA, 4.9% switched of HMA, and <1.0% received an AML-like intensive chemotherapy during the follow-up period. Treatment patterns were similar among patients initiated on AZA or DEC (Table).
Conclusions: In this US real-world observational study using claims data, risk of death or AML transformation among patients with MDS was high despite HMA treatment, especially for those with higher-risk MDS. More than 70% of patients with MDS treated with HMA were classified with higher-risk MDS. These patients had a poor prognosis, with a median OS shorter than 1 year and a time-to-AML transformation of less than 2 years from HMA initiation. These data highlight the unmet medical needs of patients with MDS treated with HMA, particularly for those in the higher-risk MDS group.
Stein:Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma US, Inc: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Membership on an entity's Board of Directors or advisory committees; Bioline: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Latremouille-Viau:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Joseph:Amgen: Equity Ownership; Cigna: Equity Ownership; Pfizer: Equity Ownership; Novartis: Employment, Equity Ownership. Shi:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Guerin:AbbVie: Other: employee of Analysis Group, Inc., which has received consultancy fees from AbbVie. Wu:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Bonifacio:Novartis: Employment. Cao:Novartis: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal