Background: Episodes of acute pain caused by vaso-occlusive crises (VOCs) are a frequent and debilitating complication associated with sickle cell disease (SCD) and represent the most common cause for emergency room (ER) visits and inpatient (IP) stays. VOCs are due to a complex pathophysiology including multicellular adhesion. The purpose of this study was to characterize VOCs and assess the costs of SCD for patients with commercial insurance using an Excel-based model.
Methods: Patients with SCD aged ≥16 years were identified in the IBM Truven MarketScan commercial databases (01/01/2000-06/30/2018). The index date was randomly selected among potential calendar dates to have ≥12 months of continuous health plan enrollment before (pre-index period) and after (follow-up period) that date. Patients with Medicare Supplemental coverage or stem cell transplant (SCT) were excluded. Data were analyzed at the state level for 23 key states with the highest concentration of patients with SCD and at the national level to be incorporated into an Excel-based model.
The key variables in the model were age, gender, state of residence, and SCD clinical characteristics measured during the 12-month pre-index period. The following model inputs were assessed during the subsequent 12-month follow-up period: number, type, and setting (i.e., IP, ER, or outpatient [OP]) of VOC episodes; and total all-cause and SCD-related healthcare costs. Costs were reported in 2018 USD from a payer's perspective. We defined a VOC episode requiring medical services in claims data as follows: medical service claims with a VOC-related diagnosis occurring within 3 days of each other, IP re-admission within 14 days of a previous IP stay (both with VOC-related diagnoses), or any follow-up medical services with VOC-related diagnoses in the 7 days following an initial VOC diagnosis. A complicated VOC type was defined as a VOC episode with a diagnosis of priapism, splenic sequestration, acute hepatic sequestration, or acute chest syndrome. Variables were stratified by annual number of VOCs (i.e., 0, 1, ≥2 VOCs) and medical service setting (i.e., IP, ER, or OP).
Results: A total of 16,092 commercially-insured patients with SCD from all US states were included in this study: mean age was 36.7 years, and 61.4% were females. In total, 27.7% had Hb-SS, 23.4% Hb-SC, 25.8% Hb-thalassemia, and 23.1% had an unspecified SCD type. The five states that contributed the highest number of patients with SCD were New York (n=1,711; 10.6%), Texas (n=1,593; 9.9%), Florida (n=1,397; 8.7%), Georgia (n=1,382; 8.6%), and California (n=966; 6.0%).
In a given year, 64.7% of patients did not have any VOC episodes, 14.0% had only 1 VOC, and 21.2% had ≥2 VOCs (10.1% had ≥4 VOCs). Among patients with ≥1 VOC, the mean number of VOC episode was 3.3 (7.3% were complicated VOCs); among those with ≥2 VOCs, this figure was 4.8 (6.9% were complicated VOCs).
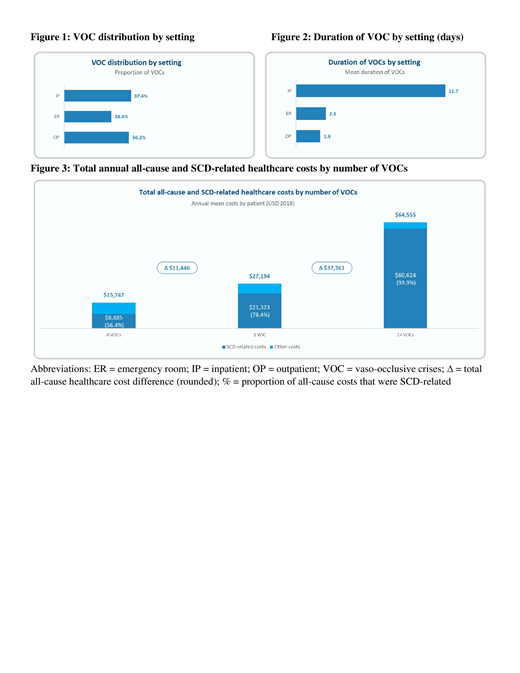
The model showed that VOC episodes were distributed as follows: 37.4% in an IP setting, 26.4% in an ER, and 36.2% in an OP setting (Figure 1). The mean duration of a VOC episode was 11.7 days in an IP setting, 2.3 days in an ER setting, and 1.9 days in an OP setting (Figure 2). Total annual all-cause healthcare costs for patients with 0, 1, and ≥2 VOCs were $15,747, $27,194, and $64,555, respectively (Figure 3). Total annual SCD-related healthcare costs for patients with 0, 1, and ≥2 VOCs were $8,885, $21,323, and $60,624, respectively, representing 56.4%, 78.4%, and 93.9% of total annual all-cause healthcare costs, respectively (Figure 3).
Conclusions: Among commercially-insured patients with SCD in the US, over one-third of patients experienced VOCs. The model showed that the contribution of SCD-related costs to annual total healthcare costs increases with the number of VOCs per year.
Joseph:Amgen: Equity Ownership; Novartis: Employment, Equity Ownership; Pfizer: Equity Ownership; Cigna: Equity Ownership. Latremouille-Viau:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Sharma:NOVARTIS HEALTHCARE PVT. LTD.: Employment. Gagnon-Sanschagrin:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Bhor:Novartis: Employment, Equity Ownership. Khare:HEALTHCARE PVT. LTD.: Employment. Singh:NOVARTIS HEALTHCARE PVT. LTD.: Employment. Serra:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Davidson:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Guerin:Novartis: Other: I am an employee of Analysis Group, Inc., which provided paid consulting services to Novartis for the conduct of this study. Shah:GBT: Research Funding; Alexion: Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal