Introduction:
Spleen tyrosine kinase (Syk), a cytoplasmic tyrosine kinase, is a member of the non-receptor type protein kinase family. Apart from the hematopoietic cells, it is also expressed in the epithelial and endothelial cells. Fostamatinib, a Syk inhibitor, has been proven beneficial in autoimmune disease like rheumatoid arthritis (RA) and immune thrombocytopenia (ITP). Murine models have shown a direct correlation between hypertension and level of R406, the active metabolite of fostamatinib. In this study, we sought to examine the cardiovascular profile of fostamatinib in published and unpublished randomized controlled trials.
Methods:
A systematic search of Pubmed, Medline and Scopus databases were done from inception till date to identify all phase 2 and 3 clinical trials of fostamatinib in patients with ITP and RA by two independent reviewers. Trials were included if they reported side effects including but not limited to cerebral ischemia or infarction, myocardial ischemia and myocardial infarction, hypertension and cardiac rhythm disorders. 35 trials were retrieved in the initial search which were excluded according to PRISMA (Preferred Reporting Items for Systematic review and Meta-Analyses) guidelines. 11 trials met the eligibility criteria and were included in the final analysis. All statistical analysis was carried out using OpenMetaAnalyst software. Categorical variables from each study are presented as proportions. The proportions from each study were subjected to arcsine transformation and pooled using a random-effects model. This yielded the pooled estimate with 95% confidence intervals. The I2 statistic was used to assess heterogeneity and a value of I2 = 25%-50% was considered mild, 50%-75% as moderate, and > 75% as severe. A P value of <0.05 was considered significant in all cases.
Results:
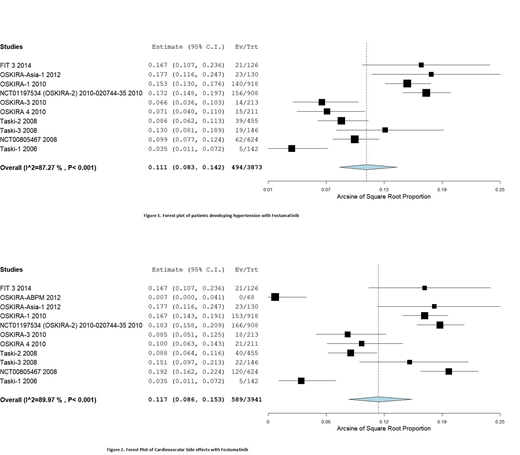
11 trials included in final study had 3941 patients. Cardio-vascular side effects were reported by all trials which ranged from 0.7% to 19.2%. The pooled estimate was 11.7% (8.6%-15.3%, P<0.001 (I2=89.97%)). The most common adverse event reported was hypertension by 10 trials with pooled estimate of 11.1% (8.3%-14.2%, p<0.001(I2=87.27%). The FIT-3 trial reported hypertension as the sole cardiovascular adverse event in 16.7% (n=21) of patients. Other adverse events reported were arrhythmias by 3 trials with incidence of 0.4% (n=11), angina was reported by 5 trials with incidence of 0.8% (n=19). Malignant hypertension was reported in 3 trials with incidence of 0.2%(n=4). 3 trials report heart failure with incidence of 0.3% (n=7). Carotid artery stenosis was observed in only one patient in Oskira 2 trial. Only 2 patients were found to have stroke from all trials, no incidence of aortic stenosis/aneurysm reported. Ventricular fibrillation and peripheral ischemia were observed in one patient respectively and circulatory shock was seen in two patients. Left bundle branch block and pericarditis were also rare side effects observed in only one patient.
Conclusion:
Our study shows that the use of fostamatinib is significantly associated with cardiovascular side effects, of which hypertension is most significant adverse event. We also recorded a few serious adverse events like hypertensive crises, myocardial infarction, coronary artery disease and congestive heart failure in few patients, but at a very low rate. Larger longitudinal studies are needed to determine the side effect profile of fostamatinib.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal