Introduction: Myelofibrosis (MF) is a rare bone marrow cancer characterized by bone marrow fibrosis and abnormal cytokine expression, often leading to splenomegaly, constitutional symptoms, and cytopenia. Prognostic scoring systems (the International Prognostic Scoring System and the Dynamic International Prognostic Scoring System) classify patients into low-, intermediate-1-, intermediate-2-, or high-risk categories. Most patients with MF have either intermediate-2- or high-risk disease, indicating a poor overall prognosis and short survival time (5-year survival rate in Europe: 35%). Ruxolitinib is the first Janus kinase inhibitor (JAKi) treatment for MF approved by the US Food and Drug Administration and the European Medicines Agency. However, the rate of discontinuation of ruxolitinib in the first 2-3 years of treatment is high (> 50%) due to treatment resistance, disease recurrence, and worsening of anemia. This literature review aimed to assess overall survival (OS) in patients with MF who have discontinued ruxolitinib.
Methods: A systematic literature review (SLR) was conducted in which Embase®, MEDLINE®, and the Cochrane Library were searched to identify published evidence from database inception until August 2018. Conference proceedings, health technology assessments, and bibliographies were also searched. Additionally, this SLR was updated in a targeted manner using Embase® until February 2019 to identify and update the OS evidence among patients with MF who have discontinued ruxolitinib. Retrieved studies were included if they were published in English and reported OS data in the targeted patient population of interest. Two independent reviewers assessed the studies against pre-defined eligibility criteria (Table 1) to include or exclude the studies, and any uncertainty was resolved by a third independent reviewer, in the case of the SLR, or by mutual agreement, in the case of the update. All extracted data were quality checked by a second independent reviewer. A descriptive, qualitative analysis was conducted to assess OS in patients with MF who have used and discontinued ruxolitinib.
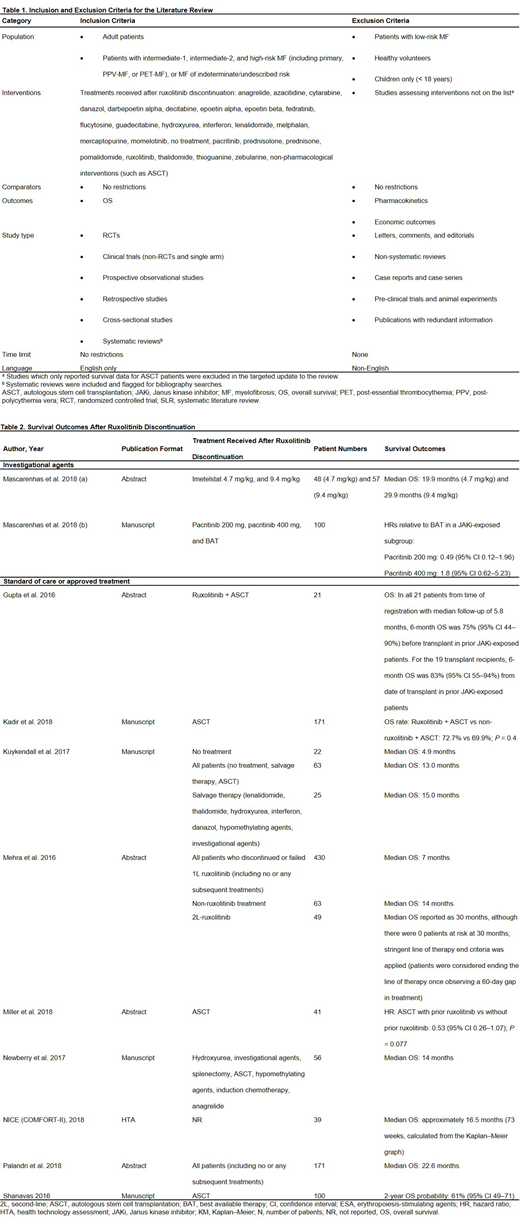
Results: Of the 4,011 publications retrieved, 11 studies were included (Table 2). Six were retrospective observational studies, 2 were randomized controlled trials (RCTs), and 3 were non-RCTs. Across all the included studies, 5 reported estimates of median OS. Across the 4 studies reporting median OS for standard of care or approved treatments, median OS ranged from 4.9-30 months (Kuykendall et al. 2017, Mehra et al. 2016, Newberry et al. 2017, Palandri et al. 2018). Patients receiving no treatment after ruxolitinib had a median OS of 4.9 months (Kuykendall et al., 2017). Median OS in patients who received treatment with salvage therapy or conventional agents (e.g. hydroxyurea, danazol, anagrelide) was typically around 14 months (14, 14, and 15 months in Mehra et al. 2016, Newberry et al. 2017, and Kuykendall et al. 2017, respectively). Estimated median OS following ruxolitinib discontinuation for early discontinuers and spleen responders in the COMFORT-II study was approximately 16.5 months (NICE, 2015). One study reported median OS for an investigational agent (Mascarenhas et al. 2018); median OS was 19.9 and 29.9 months for imetelstat 4.7 mg/kg and 9.4 mg/kg, respectively.
Conclusions: This literature review revealed that patients with MF generally experience poor OS after discontinuing ruxolitinib, especially in patients who receive no further treatments. Line of therapy definitions were rarely reported across studies, which may contribute to variations across study findings. In addition, survival estimates after prior ruxolitinib therapy varied depending on the treatment received and the reason for discontinuation of ruxolitinib. Limited survival data for investigational therapies were available from early-stage trials and may be subject to substantial variations in large-scale registrational trials. Some of the studies included in this literature review may be ongoing as they are currently available in abstract form only, and new data may become available in the near future. Sustained efforts to develop more effective treatments for patients with MF who have discontinued ruxolitinib are imminently needed.
Tang:Celgene Corporation: Employment, Equity Ownership. Taneja:BresMed Health Solutions Ltd: Employment. Rajora:BresMed Health Solutions Ltd: Employment. Sly:BresMed Health Solutions Ltd: Employment. Davison:BresMed Health Solutions Ltd: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal