Introduction: Invasive fungal infections (IFIs) are a major cause of morbidity and mortality in patients undergoing induction chemotherapy for acute myeloid leukemia (AML). During induction chemotherapy for AML patients, antifungal prophylaxis (AP) has been associated with a decreased incidence of IFIs and increased survival. However, some centers do not use AP, as it is unclear whether it improves outcomes in settings where the incidence of IFIs is low (<8% of mold infections), as at our center. We retrospectively assessed the differences in clinical outcomes and resource utilization in patients undergoing induction chemotherapy for AML before and after implementation of an AP policy and a dedicated inpatient malignant hematology service at our institution.
Methods: AML patients ≥18 years of age who underwent induction chemotherapy between 1/1/07 and 4/1/19 were identified by pharmacy records. Data was verified and extracted by two members of the research team. Primary endpoints were in-hospital mortality during induction chemotherapy and rates of fungal infections within 90-days after induction. Secondary endpoints were 30-day post induction and 90-day post-discharge survival, rates of proven/probable IFI by application of the Mycoses Study Group definitions, diagnostic resource utilization and tolerability. Categorical variables were compared with x2 or Fisher's exact tests and continuous variables with independent samples t-test or the non-parametric Mann-Whitney test as appropriate. Post-induction survival was also analyzed with Kaplan-Meier (KM) curves.
Results: 108 adult AML patients were retrospectively reviewed. Fifty-six received AP during induction and 52 did not, without significant differences in demographic or leukemia-specific factors. AP was associated with numerically less fungal infections (9/56 (16%) vs. 14/52 (27%), P=0.169) and proven/probable IFIs (3/52 (6%) vs. 0/56, P=0.1). AP was also associated with lower in-hospital (11/52 (21%) vs. 4/56 (7%), P=0.035) mortality.
Clinicians obtained more electrocardiograms (median 4 vs. 1.5, P<0.001) in the AP group; otherwise, there were no significant differences in the number of imaging studies, bronchoscopies, blood cultures, β-D-glucan and Aspergillus galactomannan tests, or adverse events, specifically clinically significant QT prolongation, nephrotoxicity or hepatotoxicity. Antifungals were more likely to be started in the non-prophylaxis group (36/52 (69%)) than changed in the prophylaxis group (23/56 (41%), P=0.003).
Implementation of a dedicated inpatient malignant hematology service led to standardization of practices including AP (24/76 (32%) vs. 32/32 (100%), P<0.001) and was associated with significantly lower in-hospital (14/76 (18%) vs. 1/32 (3%), P=0.036) and 90-day post-discharge (16/71 (23%) vs. 1/29 (3%), P=0.021) mortality.
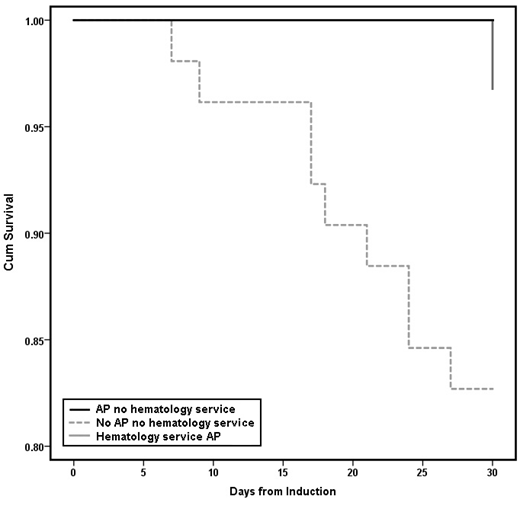
Before implementation of this service, AP was associated with numerically lower in-hospital mortality during induction (3/24 (13%) vs. 11/52 (21%), P=0.366), and significantly improved 30-day post-induction survival (Fig. 1, log-rank P=0.042).
Conclusions: In a setting where mold infections are rare, AP in patients undergoing induction chemotherapy for AML was associated with improved survival, empiric or targeted antifungal treatment, and a signal for decreased frequency of fungal infections, without substantial increases in utilization of diagnostic resources.
This study is limited in its single-center retrospective study design, the overall small number of patients, and is confounded by simultaneous changes in practice, especially with the implementation of a dedicated malignant hematology service. Notably however there was improved 30-day mortality in patients on AP versus those not on AP prior to the implementation of the malignant hematology service.
Our findings indicate that outcomes are optimized when adult patients with AML receive their care by a dedicated malignant hematology team, with implications for low-volume institutions and community practices, where AML inductions are not always performed under the care of malignant hematology specialists. Our results suggest that AP and admitting or transferring adult AML patients to a center with a dedicated malignant hematology service, when feasible, may lead to better outcomes.
Farmakiotis:Astellas: Research Funding; Viracor-Eurofins: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal